中国盐业协会网 转载-政策法规栏目 翻译
壹佰编译:英文原版应为具有约束力的真实版本(标题按中文阅读习惯略有调整)
——————————
点击进入:
https://www.x-mol.com/paper/1294139599263244288

阅读原文:
https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2018.5374

欧盟食品安全局 刊物
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS)
Re-evaluation of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536) and calcium ferrocyanide (E 538) as food additives
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Maged Younes, Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert-Remy, Gunter Georg Kuhnle, Claude Lambré, Jean-Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens-Berendsen, Matthew Wright, Alessandro Di Domenico, Henk Van Loveren, Alessandra Giarola, Zsuzsanna Horvath, Federica Lodi, Rudolf Antonius Woutersen
First published: 25 July 2018 https://doi.org/10.2903/j.efsa.2018.5374Citations: 4
Correspondence: fip@efsa.europa.eu
Requestor: European Commission
Question numbers: EFSA-Q-2011-00676, EFSA-Q-2011-00677, EFSA-Q-2011-00678
Panel members: Peter Aggett, Fernando Aguilar, Riccardo Crebelli, Birgit Dusemund, Metka Filipič, Maria Jose Frutos, Pierre Galtier, David Gott, Ursula Gundert-Remy, Gunter Georg Kuhnle, Claude Lambré, Jean-Charles Leblanc, Inger Therese Lillegaard, Peter Moldeus, Alicja Mortensen, Agneta Oskarsson, Ivan Stankovic, Ine Waalkens-Berendsen, Rudolf Antonius Woutersen, Matthew Wright and Maged Younes.
Acknowledgements: The ANS Panel wishes to acknowledge all European competent institutions, and Member State bodies that provided data for this scientific output.
Note to the amendment: The correction made regards the addition of “00” before each of the three question numbers (on page 1). To avoid confusion, the original version of the opinion has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Adopted: 29 June 2018
Amended: 30 July 2018
欧盟食品安全局(EFSA)食品添加剂和食品中添加营养来源科学小组
重新评估食品添加剂亚铁氰化钠(E 535)、亚铁氰化钾(E 536) 和亚铁氰化钙(E 538)安全性的科学意见
(在线版)
首次出版: 2018 07 25 https://doi.org/10.2903/j.efsa.2018.5374
申请人: 欧洲联盟委员会
议题编号:EFSA-Q-2011-00676, EFSA-Q-2011-00677, EFSA-Q-2011-00678
欧洲食品安全局(EFSA)食品添加剂和食品中添加营养来源科学小组(ANS)成员:彼得·阿格特,费尔南多·阿吉拉尔,里卡多·克雷贝利,比吉特·杜塞蒙德,梅特卡·菲利皮奇,玛丽亚·何塞·弗鲁托斯,皮埃尔·加尔埃,大卫·戈特,乌尔苏拉·冈德特-雷米,冈特·乔治·库恩勒,克劳德·兰布,让-查尔斯·勒布朗,因格·特蕾莎·利勒加德,彼得·莫尔德乌斯,阿利贾·滕森,阿格内塔·奥斯卡松,伊万·斯坦科维奇,伊内·瓦尔肯斯-贝伦登,鲁道夫·安托努斯·乌特森,马修·赖特和马吉德·尤尼斯。
致谢:ANS感谢所有提供本科学成果所需数据的欧洲主管机构和成员国机构。
修正的说明: 作出的更正涉及在三个问题数(第1页)之前各增加“00”。 为避免混淆,意见的原始版本已从EFSA期刊上删除,但可根据请求提供,显示所有修改的版本也是如此。
通过日期:2018年6月nt29日
修正日期:2018年7月nt30日

简目
抽象的
概括的
1 引言
2 数据和方法
3 评估
4 结论
向欧洲食品安全局提供的文件
支持信息
参考资料
缩略语
附录 A - 行业提供的 E535-538 亚铁氰化物报告使用水平摘要(mg/kg 或 mg/L,视情况而定)
附录 B - 2013 年至2018 年4月期间,标有E 535-538亚铁氰化物的食品数量和百分比占Mintel GNPD中每个食品子类别的食品总数
附录 C - 可用编号欧洲国家儿童和成人每日尿钠排泄量及其用于暴露评估的估计盐摄入量的数据
附录D - 在最高水平暴露情景和每个人群和调查的精制盐接触评估情景下,亚铁氰化物用作食品添加剂的总估计接触量摘要:平均和高暴露量(毫克/公斤 体重/天)
引用文献
Abstract
The Panel on Food Additives and Nutrient Sources added to Food (ANS) provided a scientific opinion re-evaluating the safety of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and evaluating the safety of calcium ferrocyanide (E 538) as food additives. The Panel considered that adequate exposure and toxicity data were available. Ferrocyanides (E 535–538) are solely authorised in two food categories as salt substitutes.
To assess the dietary exposure to ferrocyanides (E 535–538) from their use as food additives, the exposure was calculated based on regulatory maximum level exposure assessment scenario (maximum permitted level (MPL)) and the refined exposure assessment scenario.
Dietary exposure to ferrocyanides was calculated based on mean and high levels consumption of salts in both the regulatory maximum level and the refined scenario. In the MPL scenario, the exposure to ferrocyanides (E 535–538) from their use as a food additive was up to 0.009 mg/kg body weight (bw) per day in children and adolescents.
In the refined estimated exposure scenario, the exposure was up to 0.003 mg/kg bw per day in children and adolescents. Absorption of ferrocyanides is low and there is no accumulation in human. There is no concern with respect to genotoxicity and carcinogenicity. Reproductive studies were not available, but a no observed adverse effect level (NOAEL) of 1,000 mg sodium ferrocyanide/kg bw per day (highest dose tested) was identified from a prenatal developmental toxicity study.
The kidney appeared to be the target organ for ferrocyanides toxicity and 4.4 mg sodium ferrocyanide/kg bw per day was identified as the NOAEL for the renal effects in a chronic (2-year) study in rats. Assuming that the toxicity of this compound is due to the ferrocyanide ion only, the Panel established a group acceptable daily intake (ADI) for sodium, potassium and calcium ferrocyanide of 0.03 mg/kg bw per day expressed as ferrocyanide ion. The Panel concluded that ferrocyanides (E 535–538) are of no safety concern at the current authorised use and use levels.
抽象的
EFSA 食品添加剂和食品中添加营养来源科学小组(ANS)提供了一份科学意见,重新评估了亚铁氰化钠(E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538)作为食品添加剂的安全性。科学小组认为,亚铁氰化物(E535-538)有足够摄入量和毒性数据,获准授权在盐和盐替代品两个食品类别中作为食品添加剂。
为了评估亚铁氰化物(E 535-538)用作食品添加剂的膳食暴露量,根据监管最高水平暴露评估情景(最大使用量 MPL)和精制盐暴露评估情景计算暴露量。
膳食摄入亚铁氰化物是根据监管最大使用量和精制盐情景中的平均和高水平摄入量计算的。在MPL情景中,儿童和青少年因用作食品添加剂而暴露于亚铁氰化物(E 535-538)的剂量高达每天0.009 mg/kg bw。在精制盐评估的暴露情景中,儿童和青少年的暴露量高达每天0.003 mg/kg bw。
亚铁氰化物的吸收率低,在人体内无蓄积,无需担心遗传毒性和致癌性。虽没有生殖研究,但从一项产前发育毒性研究中确定的亚铁氰化钠 1,000 mg/kg bw/per day(测试的最高剂量),未观察到不良反应水平NOAEL(未观察到有害作用的剂量)。
肾脏似乎是亚铁氰化物毒性的靶器官,在一项对大鼠进行的持续2 年研究中,每天亚铁氰化钠4.4 mg/kg bw被确定为肾脏影响的NOAEL(未观察到有害作用的剂量)。假设该化合物的毒性仅由亚铁氰化物离子引起,科学小组确定了亚铁氰化物钠、钾和钙的每日容许摄入量(ADI)为0.03mg/kg bw,以亚铁氰化物离子表示。评估小组得出结论,亚铁氰化物(E 535-538)在目前的授权使用和使用量上不存在安全问题。
Summary
The present opinion deals with the re-evaluation of the safety of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and evaluation of the safety of calcium ferrocyanide (E 538) as food additives.
Sodium, potassium and calcium ferrocyanides (E 535, E 536 and E 538) are authorised as food additives in the European Union (EU) in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/20121.
In the EU, sodium and potassium ferrocyanide, used as food additives, were previously evaluated by the Scientific Committee on Food (SCF) in 1990. In that evaluation, the SCF agreed with the acceptable daily intake (ADI) of 0.025 mg/kg body weight (bw) per day (calculated as sodium ferrocyanide) established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) for sodium and potassium ferrocyanide.
Sodium, potassium and calcium ferrocyanide were evaluated by JECFA in 1969, 1973 and 1974. A temporary acceptance of 0–0.00125 mg/kg bw per day was established in 1969 based on a dietary level of 0.05% sodium ferrocyanide and subsequently a temporary ADI of 0–0.025 mg/kg bw per day was established. In 1974, the temporary ADI of 0–0.025 mg/kg per bw (calculated as sodium ferrocyanide)was confirmed. A larger uncertainty factor (1,000) than the generally one employed was used to compensate for the absence of a long-term feeding study.
Potassium and sodium ferrocyanide were evaluated by the UK Committees on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) in 1994 and set a group for ferrocyanides of 0–0.05 mg/kg bw per day.
The Scientific Committee for Animal Nutrition (SCAN) evaluated the safety for the target animals, the users, the workers, the consumers and the environment of sodium and potassium ferrocyanide used as anticaking agents. It was concluded that sodium and potassium ferrocyanide in salt for feed use (20, 80 and 100 mg/kg in salt for man, poultry and livestock, respectively) is acceptable in regard to the safety for target animals and human consumers.
概括的
本意见涉及重新评价亚铁氰化钠(E535)、亚铁氰化钾(E536)和亚铁氰化钙(E538)作为食品添加剂的安全性。
根据关于食品添加剂的法规(EC)第 1333/2008 号附件 II,亚铁氰化钠、钾和钙(E535、E536 和 E538)在欧盟(EU)被授权为食品添加剂,欧盟委员会法规(EU)No 231/20121中规定了具体的纯度标准。
在欧盟,用作食品添加剂的亚铁氰化钠和亚铁氰化钾曾于1990年由食品科学委员会(SCF)进行评价。在评估中,SCF同意粮农组织/世卫组织食品添加剂联合专家委员会(JECFA) 为亚铁氰化钠和亚铁氰化钾设立的每日容许摄入量ADI为0.025mg/kg。
1969年、1973年和1974年,JECFA对亚铁氰化钠、钾和化钙进行了评价。1969年,根据0.05%亚铁氰化钠的膳食水平,确定了每日0-0.00125mg/kg bw的临时可接受摄入量,随后确定了每日0-0.025mg/kg bw的临时ADI。1974年,确认了0-0.025mg/kg bw(按亚铁氰化钠计算)的临时ADI。由于缺乏长期喂养研究,采用了比通常使用的更大的不确定因素(1000)。
英国食品、消费品及环境化学物毒性委员会(COT)于1994 年对亚铁氰化钾和亚铁氰化钠进行了评估,并将亚铁氰化物的每日容许摄入量ADI定为每日0 -0.05 mg/kg bw。
欧盟动物营养科学委员会(SCAN)评估了用作抗结剂的亚铁氰化钠和亚铁氰化钾对目标动物、使用者、工人、消费者和环境的安全性。结论是,就目标动物和人类消费者的安全而言,食用、饲料用盐中的亚铁氰化钠和亚铁氰化钾(人、家禽和牲畜的盐中亚铁氰化钠和亚铁氰化钾分别为 20、80 和 100 mg/kg)是可以接受的。
Sodium, potassium and calcium ferrocyanide were evaluated by a working group established by the Nordic Council of Ministers in 2000. Sodium, potassium and calcium ferrocyanide were not considered to cause a safety problem due to the very small quantities consumed.
Potassium ferrocyanide is absorbed to a limited extent from the gastrointestinal tract following oral administration to rats and in humans absorption is low (0.25–0.42%). Potassium ferrocyanide is of low acute oral toxicity. Based on the available data, the Panel considered that the use of ferrocyanides as food additives is not of genotoxic concern and that ferrocyanides are not carcinogenic. Reproductive studies were not available, but a no observed adverse effect level (NOAEL) of 1,000 mg sodium ferrocyanide/kg bw per day (highest dose tested) was identified from a prenatal developmental toxicity study.
In a 2-year study, animals frequently showed a higher cell excretion rate in urine samples compared to controls. Since the kidney is known to be the target organ for ferrocyanide toxicity, the Panel considered the increased cell excretion rate indicative for occasional, transient kidney toxicity and identified a NOAEL of 4.4 mg/kg bw per day. Based on this NOAEL of 4.4 mg sodium ferrocyanide/kg bw per day for male rats,
the Panel derived an ADI of 0.044 mg sodium ferrocyanide/kg bw per day. Assuming that the toxicity of this compound is due to the ferrocyanide ion only, the Panel established a group ADI for sodium, potassium and calcium ferrocyanide of 0.03 mg/kg bw per day expressed as ferrocyanide ion. The Panel noted that at this ADI the potential amount of free cyanide released would not be of safety concern.
To assess the dietary exposure to ferrocyanides (E 535–538) from their use as food additives, the exposure was calculated based on (1) maximum permitted level (MPL) in FC 12.1.1 ‘Salt’ set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) the mean reported use levels of salt (defined as the refined exposure assessment scenario).
The Panel decided to use salt intake data from urinary excretion studies for the assessment of exposure to ferrocyanides (E 535–538) instead of the food consumption data from the EFSA Comprehensive European Food Consumption Database as dietary surveys are commonly not considered as a good source of information in the estimation of salt intake while a more accurate way of estimation of the salt intake is a calculation from the urinary excretion of sodium.
亚铁氰化钠、钾和钙由北欧部长理事会于2000年成立的工作组进行评估。由于亚铁氰化钠、钾和钙的食用量非常少,因此被认为不会引起安全问题。
大鼠口服亚铁氰化钾后从胃肠道吸收的程度有限,而人类吸收率较低(0.25-0.42%)。亚铁氰化钾的急性口服毒性较低。根据现有数据,科学小组认为,使用亚铁氰化物作为食物添加剂不会引起基因毒性问题,而且亚铁氰化物不会致癌。没有生殖研究,但从一项产前发育毒性研究中测定结果是亚铁氰化钠1,000mg/kg/ bw/per day(测试的最高剂量),但未观察到不良反应(NOAEL没有观察到不良效应的最高剂量)。
在一项为期2年的研究中,与对照组相比,动物在尿液样本中的细胞排泄率经常更高。由于已知肾脏是亚铁氰化物中毒的靶器官,评估小组认为细胞排泄率增加表明偶尔会出现短暂的肾毒性,并测定NOAEL为亚铁氰化钠0.044mg/kg bw per day。根据雄性大鼠亚铁氰化钠每日容许摄入量4.4mg/kg bw的NOAEL,该小组得出每日容许摄入量为亚铁氰化钠0.044mg/kg bw。假设该化合物的毒性仅由亚铁氰化物离子引起,评估小组确定了亚铁氰化物钠、钾和钙的每日容许摄入量(ADI)是0.03mg/kg bw(以亚铁氰化物离子表示)。小组指出,在此每日容许摄入量下,释放的游离氰化物的潜在量不会构成安全问题。
为了评估亚铁氰化物(E 535-538)用作食品添加剂对亚铁氰化物 (E 535-538)的膳食摄入量,根据 (1) 欧盟立法中规定的 FC 12.1.1“盐”的最大使用量(MPL)(定义为监管最大使用量暴露评估情景)和(2) 报告盐的平均使用量(定义为精制盐的暴露评估情景)
科学小组决定使用尿液排泄研究中盐的摄入量数据来评估亚铁氰化物的暴露量(E 535-538),而不是EFSA 欧洲综合食品消费数据库中的食物消费数据,因为膳食调查通常不被认为是评估盐摄入量的良好信息来源,而评估盐摄入量的更准确方法是根据尿钠排泄量计算。
Dietary exposure to ferrocyanides was calculated based on mean and high levels consumption of salts in both the regulatory maximum level and the refined scenario.
In the MPL scenario, the exposure to ferrocyanides (E 535–538) from their use as a food additive was up to 0.009 mg/kg bw per day in children and adolescents. In the refined estimated exposure scenario, the exposure was up to 0.004 mg/kg bw per day in children and adolescents. Considering that the majority of the use levels in salt reported by Industry were for sodium ferrocyanide (E 535), these exposures would correspond approximately to 0.003 mg ferrocyanide ion/kg bw per day in children and adolescents in the refined exposure scenario.
The Panel considered that the uncertainties identified indicate an overestimation of the exposure to ferrocyanides (E 535–538) as food additives.
Considering that:
·in the refined exposure scenario estimated exposure to ferrocyanides (E 535–538) would correspond approximately to 0.003 mg ferrocyanide ion/kg bw per day in children and adolescents;
·absorption of ferrocyanides from the gastrointestinal tract was low, and there is no accumulation in human;
·ferrocyanides are of low acute toxicity and not mutagenic or carcinogenic;
·reproductive studies were not available, but a NOAEL of 1,000 mg sodium ferrocyanide/kg bw per day (highest dose tested) was identified from a prenatal developmental toxicity study;
·the kidney is the target organ for ferrocyanides toxicity as characterised by the high number of cells excreted in the urine in rats;
·4.4 mg sodium ferrocyanide/kg bw per day was identified as the NOAEL for this effect in a chronic (2-year) study in rats;
·assuming that the toxicity of this compound is due to the ferrocyanide ion only, the Panel established a ADI for ferrocyanide ion of 0.03 mg/kg bw per day;
·ferrocyanides (E 535–538) are only permitted as food additives in two food categories.
·The Panel concluded that ferrocyanides (E 535–538) are of no safety concern in these current authorised use and use levels.
·The Panel further concluded that the available data give reason to revise the ADI of 0.025 mg sodium ferrocyanide/kg bw per day (equivalent approximately to 0.02 mg ferrocyanide ion/kg bw per day) based on a subchronic study, to a group ADI for sodium, potassium and calcium ferrocyanide of 0.03 mg/kg bw per day expressed as ferrocyanide ion.
膳食摄入亚铁氰化物是根据监管最大使用量和精制盐情景中的平均和高水平摄入量计算的。
在MPL情景中,儿童和青少年因用作食品添加剂而暴露于亚铁氰化物(E 535-538)的摄入量高达每天0.009mg/kg bw。在使用精制盐的评估暴露量情景中,儿童和青少年的暴露量高达每天0.004 mg/kg bw。考虑到工业界报告的盐中大部分产品使用抗结剂是亚铁氰化钠(E 535),在精制盐暴露情景下,这些暴露大约相当于儿童和青少年亚铁氰化物离子0.003mg/kg bw/per day 。
科学小组认为,所确定的不确定性表明高估了作为食品添加剂的亚铁氰化物(E 535–538)的暴露量。
考虑到:
·在精制盐暴露情景中,估计的亚铁氰化物暴露量(E 535-538)大约相当于儿童和青少年每天0.003mg/kg bw亚铁氰化物离子;
·亚铁氰化物从胃肠道吸收率低,在人体内无蓄积;
·亚铁氰化物的急性毒性低,不致突变或致癌;
·没有生殖研究,但从产前发育毒性研究中确定了亚铁氰化钠(测试的最高剂量)1,000mg/kg bw/ per day的 NOAAL;
·肾脏是亚铁氰化物中毒的目标器官,其特征是大鼠尿液中排泄的细胞数量多;
·在一项对大鼠进行持续2年的研究中,每天亚铁氰化钠4.4mg/kgbww被确定为这种效果的NOAEL(没有观察到不良效应的最高剂量);
·假设该化合物的毒性仅由亚铁氰化物离子引起,因此小组确定亚铁氰离子的ADI为0.03mg/kg bw;
·亚铁氰化物(E 535-538)仅允许作为盐和代盐制品两类食品的食品添加剂。
评估小组得出结论,亚铁氰化物(E 535-538)在目前的授权使用和最大使用量下不存在安全问题。
评估小组进一步得出结论,根据一项亚慢性研究的现有数据,有理由将亚铁氰化钠每日容许摄入量ADI 0.025mg/kg bw(相当于亚铁氰离子约0.02mg/kg bw/per day),修订为每天每公斤亚铁氰化钠、钾和钙的每日容许摄入量为0.03mg/kg bw,以亚铁氰化物离子表示。
1 Introduction
The present opinion deals with the re-evaluation of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and the evaluation of calcium ferrocyanide (E 538) when used as food additives.
1.1 Background and Terms of Reference as provided by the European Commission
1.1.1 Background
Regulation (EC) No 1333/20082 of the European Parliament and of the Council on food additives requires that food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union. In addition, it is foreseen that food additives must be kept under continuous observation and must be re-evaluated by EFSA.
For this purpose, a programme for the re-evaluation of food additives that were already permitted in the European Union before 20 January 2009 has been set up under the Regulation (EU) No 257/20103. This Regulation also foresees that food additives are re-evaluated whenever necessary in the light of changing conditions of use and new scientific information. For efficiency and practical purposes, the re-evaluation should, as far as possible, be conducted by group of food additives according to the main functional class to which they belong.
The order of priorities for the re-evaluation of the currently approved food additives should be set on the basis of the following criteria: the time since the last evaluation of a food additive by the Scientific Committee on Food (SCF) or by EFSA, the availability of new scientific evidence, the extent of use of a food additive in food and the human exposure to the food additive taking also into account the outcome of the Report from the Commission on Dietary Food Additive Intake in the EU4 of 2001. The report ‘Food additives in Europe 20005’ submitted by the Nordic Council of Ministers to the Commission, provides additional information for the prioritisation of additives for re-evaluation. As colours were among the first additives to be evaluated, these food additives should be re-evaluated with a highest priority.
In 2003, the Commission already requested EFSA to start a systematic re-evaluation of authorised food additives. However, as a result of adoption of Regulation (EU) 257/2010 the 2003 Terms of References are replaced by those below.
1引言
本意见涉及亚铁氰化钠(E 535)、亚铁氰化钾(E 536)以及亚铁氰化钙(E 538)用作食品添加剂的重新评估。
1.1欧洲联盟委员会提供的基本情况和职权范围
1.1.1基本情况
欧洲议会和理事会关于食品添加剂的法规(EC)No 1333/20082,要求食品添加剂获准在欧盟使用之前必须经过欧洲食品安全局(EFSA) 的安全评估。此外,可以预见食品添加剂必须受到持续观察,并且必须由EFSA重新评估。
为此,欧盟第257/20103号法规制定了一项计划,对2009年1月20日之前欧盟已允许的食品添加剂进行重新评估。该法规还规定,根据不断变化的使用条件和新的科学信息,在必要时对食品添加剂进行重新评估。为提高效率和实际目的,应尽量按食物添加剂所属的主要功能类别,按食物添加剂组别进行再评估。
对目前批准的食品添加剂进行重新评估的优先顺序,应根据以下标准确定:自食品科学委员会(SCF)或EFSA上次评估食品添加剂以来的时间、新科学证据的可用性、食品添加剂在食品中的使用程度以及人类对食品添加剂的暴露,同时还要考虑到2001年欧盟4膳食食品添加剂摄入量委员会的报告。北欧部长理事会向欧盟委员会提交的《20005欧洲食品添加剂》报告,为重新评估添加剂的优先顺序提供了更多信息。由于色素是首批要评估的添加剂之一,因此应以最高优先级重新评估这些食品添加剂。
2003年,欧盟委员会已经要求EFSA开始对授权的食品添加剂进行系统的重新评估。但是,由于通过了(EU)257/2010法规,2003年职权范围被以下职权范围所取代。
1.1.2 Terms of Reference
The Commission asks the European Food Safety Authority to re-evaluate the safety of food additives already permitted in the Union before 2009 and to issue scientific opinions on these additives, taking especially into account the priorities, procedures and deadlines that are enshrined in the Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re-evaluation of approved food additives in accordance with the Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives.
1.2 Information on existing authorisations and evaluations
Sodium, potassium and calcium ferrocyanides (E 535, E 536 and E 538) are authorised as food additives in the European Union (EU) in accordance with Annex II to Regulation (EC) No 1333/2008 on food additives and specific purity criteria have been defined in the Commission Regulation (EU) No 231/2012.
In EU, sodium and potassium ferrocyanide, used as food additives, was previously evaluated by the Scientific Committee on Food (SCF) in 1990 (SCF, 1991). In that evaluation, the SCF agreed with the acceptable daily intake (ADI) of 0.025 mg/kg body weight (bw) per day (calculated as sodium ferrocyanide) established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) for sodium and potassium ferrocyanide. The SCF also concluded that ‘When used as a processing aid in the production of wine only small residues are found, and only small technological levels are needed as anticaking agent in salt. Therefore, the Committee has no objection, on toxicological grounds, to the continued use for these purposes’. The Panel noted that in the SCF (1991) evaluation, calcium ferrocyanide was not explicitly mentioned.
The Panel noted that the ADI of 0.025 mg/kg bw per day for sodium and potassium ferrocyanide has been calculated as sodium ferrocyanide while the maximum permitted level is expressed as anhydrous potassium ferrocyanide.
1.1.2职权范围
欧盟委员会要求欧洲食品安全局重新评估欧盟在2009年之前已经允许的食品添加剂的安全性,并就这些添加剂发表科学意见,特别考虑到2010年3月25日第 (EU) 第 257/2010号法规中规定的优先事项、程序和截止日期,该法规根据欧洲议会第1333/2008号法规(EC)设立了食品添加剂委员会,以及对批准的食品添加剂进行重新评估的计划。
1.2 有关现有授权和评估的信息
欧盟委员会要求欧洲食品安全局重新评估欧盟在2009年之前已经允许的食品添加剂的安全性,并就这些添加剂发表科学意见,特别考虑到2010年3月25日第 (EU) 第 257/2010 号法规中规定的优先事项、程序和截止日期,该法规根据欧洲议会第1333/2008号法规(EC)设立了对批准的食品添加剂进行重新评估的计划以及食品添加剂委员会。
在欧盟,用作食品添加剂的亚铁氰化钠和亚铁氰化钾曾于1990年由食品科学委员会(SCF)进行评估(SCF,1991)。在该评估中,补充营养成分小组同意联合国粮食及农业组织/世界卫生组织FAO/WHO 食物添加剂联合专家委员会((JECFA)就亚铁氰化钠和亚铁氰化钾订定的每日容许摄入量(ADI),即每天0.025mg/kg bw(以亚铁氰化钠计算)。SCF还得出结论,“当在葡萄酒生产中用作加工助剂时,只会发现少量残留物,并且作为盐中的抗结剂只需要小型技术水平。因此,委员会基于毒理学理由不反对继续用于这些目的”。科学小组指出,在SCF(1991年)评估中,没有明确提及亚铁氰化钙。
科学小组指出,亚铁氰化钠和亚铁氰化钾的每日容许摄入量(ADI)0.025mg/kg bw,以亚铁氰化钠计算,而最大使用量则以无水亚铁氰化钾表示。
Sodium, potassium and calcium ferrocyanide were evaluated by JECFA in 1969, 1973 and 1974 (JECFA, 1970a, 1974a, 1975). A temporary acceptance of 0–0.00125 mg/kg bw per day was established in 1969 based on a dietary level of 0.05% sodium ferrocyanide (calculated by JECFA to be equivalent to 25 mg/kg bw per day) not causing toxicological effects in a subchronic rat study (Unpublished study by Oser (1959), as cited by JECFA (1975)). The Panel noted that a large uncertainty factor of 20,000 (25 mg/kg bw divided by 0.00125 mg/kg bw) was used. There is no explanation in the toxicological monograph (JECFA, 1970a) or the technical report (JECFA, 1970b) why this unusually high uncertainty factor was used. In 1973, a temporary ADI of 0–0.025 mg/kg bw per day was established on the basis of the data also available for the previous evaluation in 1969 (JECFA, 1970a). However, metabolic studies in man and if necessary a long-term study in one species were required (JECFA, 1974a,b). In 1974, the temporary ADI of 0–0.025 mg/kg per bw (calculated as sodium ferrocyanide) was confirmed and the request for metabolic studies waived due to the notion that such data would only provide limited additional information and require the use of unwanted high levels of radioactive materials in human subjects. A larger uncertainty factor (1,000) than the generally one employed was used to compensate for the absence of a long-term feeding study (JECFA, 1974c).
Potassium and sodium ferrocyanide were evaluated by the UK Committees on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) in 1994 (COT, 1994a). The Committee set a group ADI for ferrocyanides of 0–0.05 mg/kg bw per day based on a NOAEL (the lowest dose tested) in a long-term rat study of 50 mg/kg and an uncertainty factor of 100.
The Scientific Committee for Animal Nutrition (SCAN) evaluated the safety for the target animals, the users, the workers, the consumers and the environment of sodium and potassium ferrocyanide used as anticaking agents (European Commission, 2001). It was concluded that sodium and potassium ferrocyanide in salt for feed use (20, 80 and 100 mg/kg in salt for man, poultry and livestock, respectively) is acceptable in regard to the safety for target animals and human consumers.
Sodium, potassium and calcium ferrocyanide were evaluated by a working group established by the Nordic Council of Ministers in 2000 (TemaNord, 2002). Sodium, potassium and calcium ferrocyanide were not considered to cause a safety problem due to the very small quantities consumed. It was noted that without long-term or reproductive studies a full toxicological evaluation would not be possible.
JECFA于1969年、1973年和1974对亚铁氰化钠、钾和钙进行了评估(JECFA,1970a、1974a、1975)。1969年,根据0.05%亚铁氰化钠的膳食水平(根据JECFA计算相当于每天25mg/kg bw)的膳食水平,暂时接受每天0-0.00125毫克的亚慢性大鼠研究(Oser(1959)未发表的研究,如JECFA(1975)引用)而定为每天0-0.00125mg/kg bw)。科学小组注意到,使用了大量不确定因素20,000(25mg/kg bw除以0.00125mg/kg bw)。毒理学专著(JECFA,1970a)或技术报告(JECFA,1970b)中没有解释为什么使用这种异常高的不确定性因素。1973年,根据1969年上一次评估的数据(JECFA,1970a),确定了每天0-0.025mg/kg bw的临时每日容许摄入量。然而,需要对人类进行代谢研究,如有必要,需要对一个物种进行长期研究(JECFA,1974a,b)。1974 年,确认了每日0-0.025mg/kg bw(以亚铁氰化钠计算)的临时ADI,并且放弃了代谢研究的要求,因为此类数据只能提供有限的附加信息,并且需要在人类受试者中使用不需要的高水平放射性物质。使用比通常采用的不确定性因素(1,000)更大的不确定性因素(1,000)来补偿长期喂养研究的缺失(JECFA,1974c)。
1994年,英国食品、消费品和环境中化学品毒性委员会(COT)对亚铁氰化钠和钾进行了评估(COT,1994a)。在一项50 mg/kg的长期大鼠研究中,根据NOAEL(测试的最低剂量)和不确定性因素100,委员会将亚铁氰化物的每日容许摄入量设定为每天0-0.05 mg/kg bw。
动物营养科学委员会(SCAN)评估了用作抗结剂的亚铁氰化钠和亚铁氰化钾对目标动物、使用者、工人、消费者和环境的安全性(欧盟委员会,2001年)。结论是,就目标动物和人类消费者的安全而言,膳食用盐和饲料用盐中的亚铁氰化钠和亚铁氰化钾(人、家禽和牲畜盐中的亚铁氰化钠和亚铁氰化钾分别为20、80和100mg/kg)是可以接受的。
亚铁氰化钠、钾和钙由北欧部长理事会于2000年设立的工作组进行评估(TemaNord,2002 年)。由于亚铁氰化钠、钾和钙的食用量非常少,因此被认为不会引起安全问题。值得注意的是,如果没有长期或生殖研究,就不可能进行全面的毒理学评估。
2 Data and methodologies
Data
The Panel on Food Additives and Nutrient Sources added to Food (ANS) was not provided with a newly submitted dossier. EFSA launched public call for data6 and, if relevant, contacted other risk assessment bodies to collect relevant information from interested parties.
The Panel based its assessment on information submitted to EFSA following the public call for data, information from previous evaluations and additional available literature up to the last Working Group (WG) meeting.7 Attempts were made at retrieving relevant original study reports on which previous evaluations or reviews were based however these were not always available to the Panel.
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database8) was used to estimate the dietary exposure.
The Mintel's Global New Products Database (GNPD) is an online resource listing food products and compulsory ingredient information that should be included in labelling. This database was used to verify the use of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536) and calcium ferrocyanide (E 538) in food products.
Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The ANS Panel assessed the safety of sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and calcium ferrocyanide (E 538) as food additives in line with the principles laid down in Regulation (EU) 257/2010 and in the relevant guidance documents: Guidance on submission for food additive evaluations by the SCF (2001) and taking into consideration the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
2 数据和方法
数据
添加到食品中的食品添加剂和营养来源科学小组(ANS)没有提供新提交的档案。EFSA 发起了公开征集数据6,并在相关情况下联系了其他风险评估机构,从相关方收集相关信息。
小组的评估基于在公众征集数据后提交给 EFSA 的信息、先前评估的信息以及截至上次工作组(WG)会议的其他可用文献。7尝试检索以前的评估或审查所依据的相关原始研究报告,但小组并不总是可以获得这些报告。
欧洲食品安全局综合欧洲食物消费数据库(综合数据库8)用于估计从食物中摄入的分量。
英敏特的全球新产品数据库(GNPD)是一个在线资源,列出了标签中应包含的食品和强制性成分信息。该数据库用于验证亚铁氰化钠(E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538)在食品中的使用情况。
方法
本意见是根据 EFSA 关于风险评估科学方面透明度的指南(EFSA 科学委员会,2009 年)中描述的原则制定的,并遵循 EFSA 科学委员会的相关现有指导文件。
ANS 小组根据法规(EU) 257/2010 和相关指导文件中规定的原则,评估了亚铁氰化钠 (E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538) 作为食品添加剂的安全性:SCF 提交食品添加剂评估指南(2001年)并考虑 2012 年提交食品添加剂评估指南(EFSA ANS 小组,2012)。
When the test substance was administered in the feed or in the drinking water, but doses were not explicitly reported by the authors as mg/kg bw per day based on actual feed or water consumption, the daily intake was calculated by the Panel using the relevant default values as indicated in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a,b,c) for studies in rodents or, in the case of other animal species, by JECFA (2000). In these cases, the daily intake is expressed as equivalent. When in human studies in adults (aged above 18 years), the dose of the test substance administered was reported in mg/person per day, the dose in mg/kg bw per day was calculated by the Panel using a body weight of 70 kg as default for the adult population as described in the EFSA Scientific Committee Guidance document (EFSA Scientific Committee, 2012a).
Dietary exposure to sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and calcium ferrocyanide (E 538) from their use as food additives was estimated combining sodium chloride dietary intake with maximum levels according to Annex II to Regulation (EC) No 1333/20089 and reported use levels submitted to EFSA following a call for data. These sodium chloride dietary intakes were calculated from sodium intake which was assessed from urinary excretion studies. These studies were collected through EFSA focal points and the members of the EFSA Food Consumption Network. Different scenarios were used to calculate exposure (see Section 3.3.1). Uncertainties on the exposure assessment were identified and discussed.
3 Assessment
3.1 Technical data
3.1.1 Identity of the substances
In ferrocyanide coordination compounds, iron has a (positive) divalent oxidation state (Fe2+): these complexes have an octahedral geometry characterised by the coordination number of 6 (Figure 1 shows a simplified chemical structure of K4[Fe(CN)6] as an example). The hexacyanoferrate(II) anion [Fe(CN)6]4– – commonly called ferrocyanide (CAS Registry No 13408-63-4) – is very stable because of the strong bonding between iron and each cyanide group. The free ferrocyanic acid H4[Fe(CN)6], or tetrahydrogen hexakiscyanoferrate (CAS Registry No 17126-47-5), is a strong tetrabasic acid when dissolved in water (Perrin, 1969; Cotton et al., 1999; Stolzenberg, 2005).
当测试物质在饲料或饮用水中施用,但作者未根据实际饲料或水消耗量明确报告剂量为每天mg/kg bw 时,小组使用 EFSA 科学委员会指导文件(EFSA 科学委员会、2012a,b,c)用于啮齿动物的研究,或者,在其他动物物种的情况下,由JECFA(2000)进行研究。在这些情况下,每日摄入量表示为当量。在成人(18 岁以上)的人体研究中,施用的测试物质剂量以毫克/人/天为单位报告,小组使用 70 公斤的体重计算剂量,作为 EFSA科学委员会指导文件(EFSA 科学委员会,2012a)。
根据第 1333/20089 号法规(EC)附件 II,结合氯化钠摄入量与最高水平的氯化钠摄入量和在征集数据后提交给EFSA的报告使用水平,评估它们用作食品添加剂时从膳食摄入亚铁氰化钠(E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538)的膳食摄入量。这些氯化钠膳食摄入量是根据钠摄入量计算的,钠摄入量是通过尿液排泄研究评估的。这些研究是通过 EFSA 联络点和 EFSA 食品消费网络的成员收集的。使用不同的场景来计算暴露(参见第3.3.1 节)。确定并讨论了暴露评估的不确定性。
3 评估
3.1 技术数据
3.1.1 物质的特性
在亚铁氰化物配位络合物中,铁具有正二价氧化态(Fe2+):这些络合物具有八面体几何形状,其特征是配位数为6(图1 显示了 K4[Fe(CN)6] 的简化化学结构作为示例)。六氰基铁酸盐 (II) 阴离子 [Fe(CN)6]4– ——通常称为亚铁氰化物(CAS 登记号 13408-63-4) ——由于铁与每个氰化物基团之间的紧密结合,因此非常稳定。游离亚铁氰酸 H4[Fe(CN)6] 或六氢氰基铁酸盐(CAS 登记号 17126-47-5)溶于水时是一种强四元酸(佩林,1969;Cotton等人,1999;施托尔岑贝格,2005年)。
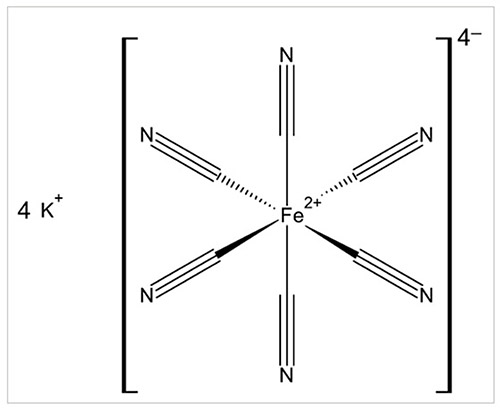
Figure 1
Simplified structural formula of potassium ferrocyanide (anhydrous form)
Sodium ferrocyanide (E 535)
According to Commission Regulation (EU) 231/201210, sodium ferrocyanide (E 535) has molecular formula Na4[Fe(CN)6] · 10H2O, EINECS (EC) No 237-081-9, and molecular weight 484.1 g/mol.
The EINECS (EC) No 237-081-9 corresponds to the CAS Registry No 13601-19-9 which is for the anhydrous form. The CAS Registry number for the hydrate form is 14434-22-1 (these identifiers are not present in the Regulation). In JECFA (2006), the chemical is identified as sodium ferrocyanide with INS No 535; the reported CAS Registry number identifies the anhydrous form.
Based mainly on Commission Regulation (EU) 231/2012, JECFA (2006), and SciFinder Online, a selection of synonyms and identifiers includes yellow prussiate of soda; sodium hexacyanoferrate; hexacyanoferrate of sodium; sodium hexacyanoferrate decahydrate; tetrasodium hexacyanoferrate(4–) decahydrate.
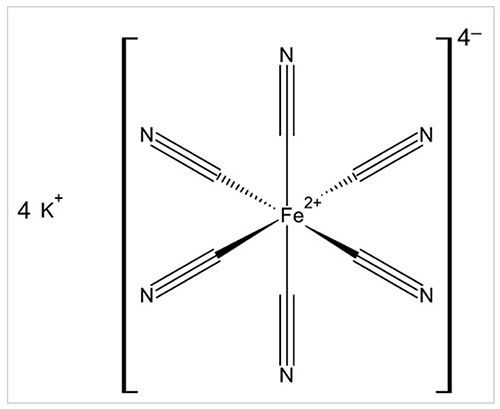
图 1
亚铁氰化钾(无水形式)的简化结构式
亚铁氰化钠(E 535)
根据欧盟委员会法规(EU)231/201210,亚铁氰化钠(E 535)的分子式为Na4[Fe(CN)6]·10H2O,EINECS(EC)编号 237-081-9,分子量484.1 g/mol。
EINECS(EC)编号237-081-9, 对应于用于无水形式的CAS登记编号13601-19-9。水合物形式的 CAS 登记号为 14434-22-1(这些标识符未出现在法规中)。在JECFA(2006)中,该化学品被鉴定为亚铁氰化钠,INS 编号为535;报告的 CAS 登记号标识了无水形式。
主要基于欧盟委员会法规(EU)231/2012、JECFA(2006)和 SciFinder 在线,精选的同义词和标识符包括:黄血盐钠;六氰基铁酸钠;六氰基铁酸钠;六氰基铁酸钠十水合物;十水合亚铁氰化钠。
Potassium ferrocyanide (E 536)
According to Commission Regulation (EU) 231/2012, potassium ferrocyanide (E 536) has molecular formula K4[Fe(CN)6] · 3H2O, EINECS (EC) No 237-722-2, and molecular weight 422.4 g/mol.
The EINECS (EC) No 237-722-2 correspond to the CAS Registry No 13943-58-3 which is for the anhydrous form. The CAS Registry number for the hydrate form is 14459-95-1 (these identifiers are not present in the Regulation). In JECFA (2006), the chemical is identified as potassium ferrocyanide with INS No 536; the reported CAS Registry number identifies the anhydrous form.
Based mainly on Commission Regulation (EU) 231/2012, JECFA (2006), and SciFinder Online, a selection of synonyms and identifiers includes: yellow prussiate of potash; potassium hexacyanoferrate; hexacyanoferrate of potassium; potassium ferrocyanide trihydrate; tetrapotassium hexacyanoferrate(4–) trihydrate.
Calcium ferrocyanide (E 538)
According to Commission Regulation (EU) 231/2012, calcium ferrocyanide (E 538) has molecular formula Ca2[Fe(CN)6] · 12H2O and molecular weight 508.3 g/mol. In JECFA (2006), the chemical is identified as calcium ferrocyanide with INS No 538. The Panel noted that, according to the database (Scifinder Online and EC Inventory of Chemicals), the EINECS (EC) No 215-476-7 and the CAS Registry No 1327-39-5 – respectively reported in the Regulation and in JECFA (2006) – refer to calcium aluminium silicate and not to calcium ferrocyanide (E 538).
The EINECS (EC) No 237-508-9 and the CAS Registry No 13821-08-4 correspond to the calcium ferrocyanide anhydrous.
Based mainly on Commission Regulation (EU) 231/2012, JECFA (2006) and SciFinder Online, a selection of synonyms and identifiers includes: yellow prussiate of lime; calcium hexacyanoferrate; hexacyanoferrate of calcium; dicalcium hexacyanoferrate(4–).
The CAS Registry and EINECS (EC) numbers reported above for the three ferrocyanides were subject to confirmatory steps to minimise the uncertainty of an equivocal identification. However, in addition to the observations brought forward for calcium ferrocyanide, the Panel also noted that the same CAS Registry and/or EINECS (EC) numbers may occasionally be found to identify marketed hydrous or anhydrous compounds.
亚铁氰化钾(E 536)
根据欧盟委员会法规(EU)231/2012,亚铁氰化钾(E 536)的分子式为 K4[Fe(CN)6]·3H2O,EINECS(EC)编号 237-722-2,分子量 422.4 g/mol。
EINECS(EC)编号 237-722-2,对应于用于无水形式的 CAS 登记编号 13943-58-3。水合物形式的 CAS 登记号为 14459-95-1(这些标识符未出现在法规中)。在JECFA(2006年)中,该化学品被鉴定为亚铁氰化钾,INS 编号为536;报告的CAS登记号标识了无水形式。
主要基于欧盟委员会法规(EU)231/2012、JECFA(2006)和 SciFinder Online,精选的同义词和标识符包括:钾黄普鲁士盐;六氰合铁酸钾;六氰高铁酸钾;三水亚铁氰化钾;四钾六氰基铁酸盐三水合物。
亚铁氰化钙(E 538)
根据欧盟委员会法规(EU)231/2012,亚铁氰化钙(E 538)的分子式为Ca2[Fe(CN)6]·12H2O和分子量508.3 g/mol。在JECFA(2006)中,该化学品被确定为亚铁氰化钙,INS 编号为538。评估小组指出,根据数据库(Scifinder Online和EC化学品清单),分别在法规和JECFA(2006)中报告的EINECS(EC)No 215-476-7和CAS Registry No 1327-39-5——指的是硅酸铝钙,而不是亚铁氰化钙(E 538)。
EINECS(EC)编号237-508-9和CAS登记编号13821-08-4,对应于无水亚铁氰化钙。
主要基于欧盟委员会法规(EU)231/2012、JECFA(2006)和 SciFinder Online,精选的同义词和标识符包括:六氰合铁(II)酸钙;钙六氰铁酸盐;六氰铁酸钙;二钙六氰铁酸盐(4–)。
上述报告的三种亚铁氰化物的 CAS 登记册和 EINECS(EC)编号需要采取确认步骤,以尽量减少鉴定模棱两可的不确定性。然而,除了针对亚铁氰化钙提出的意见外,评估小组还指出,有时可能会发现相同的CAS 登记号和/或EINECS(EC)号来识别市售的含水或无水化合物。
3.1.2 Specifications
Commission Regulation (EU) No 231/2012 lays down specifications for sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and calcium ferrocyanide (E 538) used as food additives: as the three chemicals have substantially the same specifications, the latter have been reported in Table 1 only once although in the Regulation they come in individual sections. JECFA also established specifications for the same chemicals to be used as food additives (JECFA, 2006), as shown in Table 1.
Table 1. Specifications established for sodium ferrocyanide (E 535), potassium ferrocyanide (E 536), and calcium ferrocyanide (E 538) according to Commission Regulation (EU) No 231/2012 and JECFA (2006)
Commission Regulation (EU) No 231/2012 | JECFA (2006) | |
Assay | Content not less than 99.0% of the respective ferrocyanide | Not less than 99.0% of the respective ferrocyanide |
Description
| Yellow crystals or crystalline powder for sodium and calcium ferrocyanides; lemon yellow crystals for potassium ferrocyanide | Yellow crystals or crystalline powder |
Identification Test for metal (Na, K, or Ca) Test for ferrocyanide |
Passes test of the respective ferrocyanide
Passes test |
Passes test of the respective ferrocyanide
Passes test |
Solubility | – | All soluble in water; sodium and potassium ferrocyanides insoluble in ethanol |
Purity Free oisture Water insoluble matter Chloride |
Not more than 1.0% Not more than 0.03%
Not more than 0.2% |
– –
– |
Sulfate Free cyanide Ferricyanide Lead Arsenic | Not more than 0 Not detectable Not detectable Not more than 5 mg/kg – | – Not detectable Not detectable Not more than 5 mg/kg Not more than 3 mg/kg |
3.1.2 规范
欧盟委员会法规(EU)No 231/2012 规定了用作食品添加剂的亚铁氰化钠(E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538)的规范:由于这三种化学品的规格基本相同,后者仅在表1中报告过一次,尽管在法规中它们被单独列为章节。JECFA 还为用作食品添加剂的相同化学品制定了规范(JECFA,2006 年),如表 1 所示。
表 1.根据欧盟委员会法规(EU)No 231/2012 和JECFA(2006)为亚铁氰化钠(E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538)制定的规格
表 1.根据欧盟委员会法规(EU)No 231/2012 和JECFA(2006)为亚铁氰化钠(E 535)、亚铁氰化钾(E 536)和亚铁氰化钙(E 538)制定的规格
欧盟委员会法规(EU)No 231/2012 | JECFA (2006) | |
测定 | 含量不少于其含量的99.0% | 不少于99.0%的亚铁氰化物 |
描述 | 亚铁氰化钠和亚铁氰化钙为黄色结晶或结晶粉末;亚铁氰化钾的柠檬黄色晶体 | 黄色结晶或结晶性粉末 |
鉴定 | ||
金属检测(Na、K 或 Ca) | 通过相应亚铁氰化物的测试 | |
亚铁氰化物测试 | 通过测试 | 通过测试 |
溶解性 | - | 都溶于水;亚铁氰化钠和亚铁氰化钾不溶于乙醇 |
纯度 | ||
游离水分 | 不超过1.0% | - |
水不溶物 | 不超过0.03% | - |
氯化物 | 不超过0.2% | - |
硫酸盐 | 不超过0.1% | - |
游离氰化物 | 检测不到 | 检测不到 |
铁氰化物 | 检测不到 | 检测不到 |
铅 | 不超过5mg/kg | 不超过5mg/kg |
砷 | - | 不超过3mg/kg |
The Panel noted that the solubility and the limit for arsenic are not specified in the EU specification in contrast to the JECFA specification (2006).
3.1.3 Manufacturing process
As reported by Wong-Chong and co-workers (2006), the three food additives described above are fully synthetic. Sodium ferrocyanide is produced in aqueous medium from crude sodium cyanide and ferrous sulphate according to the canonical expression:
6NaCN+FeSO4+Heat→Na4[Fe(CN)6]+Na2SO4
he sodium ferrocyanide decahydrate salt is recovered by crystallisation. The potassium salt is produced by reacting sodium ferrocyanide with calcium hydroxide and potassium chloride and carbonate according to the following reactions:
Na4[Fe(CN)6]+2Ca(OH)2→Ca2[Fe(CN)6]+4Na(OH)
Ca2[Fe(CN)6]+2K2CO3→K4[Fe(CN)6]+CaCO3
Calcium ferrocyanide is produced by reacting sodium ferrocyanide with calcium hydroxide, as visible above.
According to Stolzenberg (2005), Na4[Fe(CN)6]·10H2O is produced from calcium cyanide, iron(II) sulphate, and sodium carbonate in aqueous medium at 100°C, with a process similar to that described hereafter for the potassium derivative. K4[Fe(CN)6]·3H2O is prepared from calcium cyanide and iron(II) sulfate at a temperature above 100°C; insoluble products are removed and potassium chloride is added; the produced precipitate of calcium potassium ferrocyanide is redissolved as the potassium salt by addition of potassium carbonate; the insoluble calcium carbonate is removed and K4[Fe(CN)6]·3H2O is crystallised by rapid cooling. Ca2[Fe(CN)6] is obtained by reaction of liquid or gaseous hydrogen cyanide with iron(II) chloride in an alkaline aqueous medium (pH > 8) containing calcium hydroxide or calcium carbonate.
3.1.4 Methods of analysis in food
During the course of the long chemical history of ferrocyanides, the latter have found many diverse applications in analytical chemistry; likewise, many analytical methods have been developed for their detection in various matrices.
科学小组指出,与JECFA规范(2006年)相比,欧盟规范中没有规定砷的溶解度和限值。
3.1.3 制造过程
据 Wong-Chong 及其同事 (2006) 报告,上述三种食品添加剂是完全合成的。亚铁氰化钠由粗氰化钠和硫酸亚铁在水性介质中根据规范表达式生产:
6NaCN+FeSO4+Heat→Na4[Fe(CN)6]+Na2SO4
十水合亚铁氰化钠通过结晶回收。通过以下反应,将亚铁氰化钠与氢氧化钙和氯化钾及碳酸盐反应,按以下反应制得钾盐
Na4[Fe(CN)6]+2Ca(OH)2→Ca2[Fe(CN)6]+4Na(OH)
Ca2[Fe(CN)6]+2K2CO3→K4[Fe(CN)6]+CaCO3Ca2
亚铁氰化钙是通过亚铁氰化钠与氢氧化钙反应生成的,如上所示。
根据施托尔岑贝格(2005)的说法,Na4[Fe(CN)6]·10H2O 由氰化钙、硫酸铁(II)和碳酸钠在100°C 的水介质中生成,其过程类似于下文中描述的钾衍生物K4[Fe(CN)6·]3H2O 由氰化钙和硫酸铁(II)在 100°C 以上的温度下制备;除去不溶物,加入氯化钾;通过添加碳酸钾,将亚铁氰化钙钾产生的沉淀物重新溶解为钾盐;去除不溶性碳酸钙,快速冷却使 K4[Fe(CN)6]·3H2O 结晶。Ca2[Fe(CN)6] 由液态或气态氰化氢与氯化铁(II)在含有氢氧化钙或碳酸钙的碱性水介质(pH > 8)中反应获得。
3.1.4 食品中的分析方法
在亚铁氰化物漫长的化学历史中,亚铁氰化物在分析化学中发现了许多不同的应用;同样,已经开发了许多分析方法,用于在各种基质中检测它们。
In the paper by Roberts and Wilson (1968), ferrocyanide ([Fe(CN)6]4−) in commercial sodium chloride was determined spectrophotometrically as its iron complex in the range 0.013–50.0 mg/kg. The iron complex was concentrated from a large volume of sample solution by filtration on kieselguhr, and a reproducible Prussian Blue colour formed in a small volume under controlled conditions. Aquopentacyanoferrate ([Fe(CN)5H2O]3−), a possible albeit quite uncommon interfering substance, could be determined simultaneously, and the amounts of each complex present were thereby estimated. Some interference was caused by carbonyl pentacyanoferrate ([Fe(CN)5CO]3−), a compound unusually present whose precise determination was however achieved by using a similar principle of concentration, but with different reagents to develop the iron complex. The optical densities were measured at 700 nm for ferrocyanide, at 700 and 860 nm for aquopentacyanoferrate, and at 530 nm for carbonyl pentacyanoferrate. The procedure described in general exhibited quantitative recoveries and was suitable for ferrocyanide determination at concentrations as low as 0.10 mg/kg salt. No interference was caused by the presence of other iron-cyanogen complexes, or by the usual impurities and additives in commercial salts.
A rapid method was developed by Li et al. (2006) for the determination of trace amounts of potassium ferrocyanide (K4[Fe(CN)6]) in salted foods (eggplants) and table salt. When potassium ferrocyanide reacted with triaminotriphenylmethane dyes to form ion-association complexes, resonance Rayleigh scattering (RRS) intensities were enhanced greatly relative to the uncomplexed chemicals. Experimental trials were carried out with ethyl violet (EV), crystal violet (CV), and methyl violet (MV), the highest RRS response being obtained with EV. A spectrofluorophotometer was used for recording the RRS spectra and measuring the scattered intensity; the maximum peaks occurred at approximately 329 nm. The detection limit of the EV system was 7.8 ng/mL over the 4.8–6.8-pH range. The method showed a quantitative recovery for potassium ferrocyanide at mg/kg levels (RSD < 5%) and was considered to be suitable for the determination of trace amounts of potassium ferrocyanide in colour salted food. In a subsequent paper – also focusing on the determination of potassium ferrocyanide in salted food (eggplants and lavers) and table salt – Li et al. (2007) reported that double-charged triaminotriphenylmethane dyes (e.g. methyl green (MeG), iodine green (IG)) in acidic medium (pH 1.0) reacted with the ferrocyanide anion to form 2∶1 ion-association complexes. The latter were characterised by a change of absorption and a remarkable enhancement of RRS intensities relative to the uncomplexed chemicals. The maximum RRS wavelengths were all located at 276 nm; a spectrofluorophotometer was used for recording the RRS spectra and measuring the scattering intensity. The intensity of RRS was directly proportional to the concentration of the ferrocyanide anion in the ranges of 0.03–5.7 and 0.04–5.9 μg/mL for the MeG and IG systems, respectively. The RRS method showed a good selectivity and high sensitivity, with detection limits for potassium ferrocyanide of 9.3 and 11.2 ng/mL for the MeG and IG systems, respectively. In salted eggplant and laver samples, potassium ferrocyanide recovery was quantitative at the levels tested (low mg/kg) (RSD = 3.2–6.2%).
在罗伯茨和威尔逊的论文中(1968),通过分光光度法确定商品氯化钠中的亚铁氰化物([Fe(CN)6]4−)为其铁络合物,范围为0.013–50.0 mg/kg。通过在硅藻土上过滤,从大量样品溶液中浓缩铁络合物,并在受控条件下在小体积中形成可重现的普鲁士蓝。水合五氰铁酸盐([Fe(CN)5H2O]3−),是一种可能但相当罕见的干扰物质,可以同时测定,从而估计存在的每种络合物的量。一些干扰是由五氰基铁酸酯([Fe(CN)5CO]3−)引起的,这是一种不寻常存在的化合物,然而,其精确测定是通过使用类似的浓缩原理来实现的,但使用不同的试剂来开发铁络合物。在 700 nm 处测量亚铁氰化物的光密度,在 700 和 860 nm 处测量五氰基铁酸盐的光密度,在 530 nm 处测量羰基五氰基铁酸盐的光密度。上述程序通常表现出定量回收率,适用于浓度低至 0.10 mg/kg 盐的亚铁氰化物测定。其他铁氰基复合物的存在或商品盐中常见杂质和添加物,均不会造成干扰。
李等人(2006 年)开发了一种快速方法,用于测定咸味食品(茄子)和食盐中痕量的亚铁氰化钾(K4[Fe(CN)6])。当亚铁氰化钾与三氨基三苯基甲烷染料反应形成离子缔合物时,相对于未络合的化学物质,共振瑞利散射(RRS)强度大大增强。用乙紫(EV)、结晶紫(CV)和甲基紫(MV)进行了实验试验,EV 获得了最高的 RRS 反应。使用荧光分光光度计记录 RRS 光谱并测量散射强度;最大峰出现在大约 329 nm 处。EV 系统的检测限在 4.8 - 6.8 pH 范围内为 7.8 ng/mL。该方法显示亚铁氰化钾的定量回收率为 mg/kg 水平 (RSD < 5%),被认为适用于测定彩色盐渍食品中痕量亚铁氰化钾。在随后的一篇论文中,同样关注咸味食品(茄子和紫菜)和食盐中亚铁氰化钾的测定,李等人(2007年)报告了酸性介质(pH 1.0)中的双电荷三氨基三苯甲烷染料(例如甲基绿(MeG)、碘绿(IG))与亚铁氰化物阴离子反应形成 2∶1 离子缔合物。后者的特点是相对于未络合的化学物质吸收变化和 RRS 强度的显着增强。最大 RRS 波长均位于 276 nm;使用荧光分光光度计记录 RRS 光谱并测量散射强度。对于 MeG 和 IG 系统,RRS 的强度与亚铁氰化物阴离子的浓度成正比,分别为 0.03–5.7 和 0.04–5.9 μg/mL。RRS 方法具有良好的选择性和高灵敏度,MeG 和 IG 系统对亚铁氰化钾的检出限分别为 9.3 ng/mL 和 11.2 ng/mL。在盐渍茄子和紫菜样品中,亚铁氰化钾回收率在测试水平(低水平 mg/kg)下为定量回收率(RSD = 3.2 - 6.2%)。
A flow injection (FI) system for a sensitive determination of ferrocyanide was described by Yamane et al. (2006). The anion exchange column incorporated in the FI system was utilised for separation and preconcentration of ferrocyanide from a large excess of sodium chloride (matrix) and co-existing other substances, and for the detection reaction of ferrocyanide, adsorbed on the column, with Fe(III) complex with 1,10-phenanthroline ([Fe(o-phen)3]3+): the resultant ferroin ([Fe(o-phen)3]2+) was detected spectrophotometrically at 512 nm. The Fe(III) 1,10-phenanthroline complex was prepared in-line by passing a ferroin solution through a manganese dioxide reactor in the flow system. A linear ferrocyanide calibration over the range of 0–0.3 mg/kg in the presence of sodium chloride (0.5 mol/L) was obtained using a 6-m sample loop injection. The coefficient of variation for (potassium) ferrocyanide added to purified salt in the range of 0.050–0.200 mg/kg was better than 5%; the estimated limit of detection was 0.003 mg/kg. The FI system was successfully applied to determine ferrocyanide at mg/kg level in real salt samples with a precision better than 3% and quantitative recovery.
Lim et al. (2018) developed and validated a rapid high-performance liquid chromatography (HPLC) method to determine the presence of ferrocyanide ions ([Fe(CN)6]4–) in food grade salts (sodium chloride). An analytical column coupled with a guard column and mobile phase comprised of sodium perchlorate and sodium hydroxide were employed with a photodiode array detector set at a wavelength of 221 nm. Samples were dissolved in 0.02 M sodium hydroxide solution and filtered through a 0.22-μm polyvinylidene difluoride membrane. For processed salts including herbs and spices, a C18 cartridge was applied to minimise interference from salt matrices. The method was characterised as to linearity, accuracy (recovery), precision, limit of detection (LOD) and limit of quantification (LOQ), and measurement uncertainty. Linearity was good from 0.1 to 10 mg/L; LOD and LOQ values were determined to be 0.02 and 0.07 mg/kg, respectively. Ferrocyanide recoveries in six salt matrices – originally ferrocyanide-free, then each spiked with (sodium) ferrocyanide at 1, 5, and 10 mg/kg for the validation study – ranged from 80.3% to 103.5% (RSD = 0.3–4.4%). The method was applied to a large number of commercial products. These results indicated that the method was suitable for ferrocyanide ion determination in various food grade salts, with a good potential for application to routine analysis.
3.1.5 Stability of the substance, and reaction and fate in food
山内等人(2006年)描述了一种用于灵敏测定亚铁氰化物的流动进样(FI)系统。F 系统中掺入的阴离子交换柱用于从大量过量氯化钠(基质)和共存的其他物质中分离和预浓缩亚铁氰化物,以及用于检测吸附在色谱柱上的亚铁氰化物与与 1,10-菲咯啉([Fe(o-phen)3]3+)的复合物:在 512 nm 处用分光光度法检测所得铁蛋白([Fe(o-phen)3]2+)。Fe(III)1,10-菲咯啉配合物是通过使铁溶液通过流动系统中的二氧化锰反应器在线制备的。在氯化钠(0.5 mol/L)存在下,使用 6 m 样品定量环进样获得 0 - 0.3 mg/kg 范围内的线性亚铁氰化物校准。在0.050-0.200 mg/kg的纯化盐中添加亚铁氰化物(钾)的变异系数优于5%;估计检测限为0.003 mg/kg。FI 系统成功应用于实际盐样品中 mg/kg 水平的亚铁氰化物测定,精密度优于3%,定量回收率优于3%。
Lim 等人(2018年)开发并验证了一种快速高效液相色谱(HPLC) 方法,用于确定食品级盐(氯化钠)中亚铁氰离子([Fe(CN)6]4–) 的存在。采用与保护柱和由高氯酸钠和氢氧化钠组成的流动相联用的分析柱,以及波长设置为 221 nm 的光电二极管阵列检测器。将样品溶于 0.02 M 氢氧化钠溶液中,并通过 0.22 μm 聚偏二氟乙烯膜过滤。对于包括香草和香料在内的加工盐,使用C18小柱以最大限度地减少盐基质的干扰。该方法的特性为线性、准确度(回收率)、精密度、检测限(LOD)和定量限(LOQ)以及测量不确定度。线性在 0.1 至 10 mg/L 范围内良好;LOD 和 LOQ 值分别测定为0.02 和 0.07 mg/kg。在六种盐基质中,亚铁氰化物的回收率在80.3%至103.5%之间RSD =0.3 - 4.4%),最初不含亚铁氰化物,然后在验证研究中分别加标1、5和10 mg/kg的亚铁氰化钠。该方法被应用于大量商品盐。这些结果表明,该方法适用于测定各种食品级盐中的亚铁氰化物离子,具有良好的常规分析潜力。
3.1.5 物质的稳定性、在食品中的反应和命运
Kruse and Thibault (1973) investigated the decomposition of ferro- and ferricyanide (K4[Fe(CN)6] and K3[Fe(CN)6]) as a function of pH, illumination, and temperature. For routine measurements of hydrogen cyanide (HCN), the separation of the free acid from the sample by means of diffusion in Conway microdiffusion cells was employed; the final measurements were carried out by titration or colorimetry. Experiments were conducted to measure the transport of hydrogen cyanide as a function of pH and time: complete recovery of free cyanide was obtained at pH 7 or lower, in diffusion periods of ~ 5 h. At pH higher than 9, recovery decreased; at pH below 5, the decomposition of complex cyanides was more significant. Complex cyanides decomposed only very slowly, if at all, above pH 5 in the dark; however, the normal tungsten or fluorescent light and lower pH greatly accelerated the decomposition.
Storage life of meat and meat products is often limited by oxidative processes (such as colour changes from red to brown/grey and/or development of rancid taste); therefore, factors influencing oxidative changes are of great interest to meat manufacturers. Influence of salt (NaCl) and potassium ferrocyanide (K4[Fe(CN)6]) on oxidative stability of minced pork meat was investigated by Hansen et al. (1996). Ferrocyanide was found to affect lipid oxidation in the frozen (−22°C) meat both in usual concentrations (≤ 0.4 mg/kg meat), when added together with food grade salt to yield 2% salt in the product, and in unusually high concentrations (≥ 80 mg/kg meat) added separately or together with chemically pure sodium chloride. The level of ferrocyanide obtained from adding 2% salt accelerated the development of lipid hydroperoxides, but affected the development of thiobarbituric acid-reactive substances (TBARS) to a lesser degree; high levels of ferrocyanide seemed to protect hydroperoxides from degradation to secondary lipid oxidation products (measured as TBARS).
Addition of ferrocyanide in high concentrations resulted in immediate discoloration of the meat independent of the presence of 2% salt, whereas products with added commercial table salt (ferrocyanide at level of 7 mg/kg salt), products with added pure sodium chloride, and products without additives did not discolour immediately. However, after approximately 3 weeks of storage all products discoloured at a similar rate possibly due to a relevant contribution from background colour, reflecting colour changes just below the surface of products. Products with high concentrations of ferrocyanide were observed to have become red within the product. Products added the highest ferrocyanide concentration (17,500 mg/kg meat) became redder at the surface between the first and third week of storage (overall storage duration, 56 days). This was thought to support the suggestion that ferrocyanide was oxidised to ferricyanide ([Fe(CN)6]3–) during storage under the experimental conditions adopted, as ferricyanide was a red complex and could contribute to the colour of the product. A mechanism involving the ferrocyanide-ferricyanide redox couple of pigment-catalyzed lipid oxidation was suggested, based on an observed correlation between oxymyoglobin oxidation (measured as tristimulus colorimetry) and lipid oxidation (measured as TBARS).
Kruse和Thibault(1973)研究了亚铁和铁氰化物(K4[Fe(CN)6] 和 K3[Fe(CN)6])的分解与 pH 值、光照和温度的关系。对于氰化氢(HCN)的常规测量,采用在Conway微扩散池中扩散从样品中分离游离酸;最终测量通过滴定法或比色法进行。进行了实验以测量氰化氢的迁移与 pH 值和时间的关系:在 pH 值为 7 或更低时,在 ~5 h的扩散期内,获得游离氰化物的完全回收。当 pH 高于 9 时,回收率降低;在 pH 值低于 5 时,络合氰化物的分解更为显著。复合氰化物在黑暗中分解速度非常慢,如果有的话,在 pH 值 5 以上;然而,正常的钨或荧光灯和较低的 pH 值大大加速了分解。
肉类和肉制品的储存寿命通常受到氧化过程的限制(例如颜色从红色变为棕色/灰色和/或产生酸败味)。因此,影响氧化变化的因素对肉类制造商非常感兴趣。Hansen 等人(1996)研究了盐(NaCl)和亚铁氰化钾(K4[Fe(CN)6])对猪肉末氧化稳定性的影响。研究发现,亚铁氰化物会影响冷冻(-22°C)肉类中的脂质氧化,无论是正常浓度 (≤ 0.4 mg/kg 肉类)、与食品级盐一起添加以产生2% 的盐,以及异常高浓度(≥ 80 mg/kg 肉类)单独添加或与化学纯氯化钠一起添加。添加 2% 盐获得的亚铁氰化物水平加速了脂质氢过氧化物的开发,但对硫代巴比妥酸反应物质(TBARS)的开发影响较小。高水平的亚铁氰化物似乎可以保护氢过氧化物不降解为二次脂质氧化产物(以TBARS 测量)。
添加高浓度的亚铁氰化物会导致肉立即变色,而不受 2% 盐的存在影响,而添加商品食盐的产品(盐含量为7 mg/kg的亚铁氰化物)、添加纯氯化钠的产品和无添加剂的产品不会立即变色。然而,在储存大约 3 周后,所有商品都以相似的速度变色,这可能是由于背景颜色的相关贡献,反映了商品表面下方的颜色变化。观察到高浓度亚铁氰化物的产品在产品中变成了红色。添加量最高的亚铁氰化物浓度(17,500 mg/kg肉类)的产品在储存的第一周和第三周之间(总储存时间,56 天)在表面变红。这被认为支持了亚铁氰化物在所采用的实验条件下在贮存过程中被氧化成铁氰化物([Fe(CN)6]3–)的说法,因为铁氰化物是一种红色络合物,可以影响产品的颜色。基于观察到的氧合肌红蛋白氧化(以三刺激比色法测量)和脂质氧化(以 TBARS 测量)之间的相关性,提出了一种涉及色素催化脂质氧化的亚铁氰化物-铁氰化物氧化还原对的机制。
Nguyen et al. (2012) investigated the effects of added potassium ferrocyanide (K4[Fe(CN)6]) in different concentrations (2.5, 7.5, and 100 mg/kg) in salt on lipid oxidation in cod during salting, storage (up to 6 months, at ~ 2°C), and rehydration. An increase in ferrocyanide concentration accelerated lipid oxidation of the salted cod, as observed by increases in lipid hydroperoxides (PV) and TBARS, as well as in the development of fluorescence compounds: the fluorescence shift (δF) was determined in both the organic-chloroform phase (δFOR) and the aqueous-methanol phase (δFAQ) from extraction processes. A yellow discolouration (higher b* value, b* being an indicator of yellowness) of salted cod was associated with higher levels of oxidation derivatives. High correlations were found between PV, TBARS and free fatty acids (FFA), as well as between FFA and δFOR. The results of principal component analysis showed that TBARS, b* value and δFOR were the strongest indicators of lipid oxidation during salting and storage.
Dorazio and Bruckner (2015) presented findings on the mode of action of submonoatomic layers of sodium ferrocyanide on sodium chloride crystals, to act as an anticaking agent through nucleation inhibition. Sodium ferrocyanide on store-bought table salt could be readily detected due to the appearance of an intense blue-green colour following formation of Prussian Blue (Fe4[Fe(CN)6]3·nH2O) upon addition of slightly yellow aqueous iron(III) trichloride.
3.2 Authorised uses and use levels
Maximum levels of Ferrocyanides (E 535–538) have been defined in Annex II to Regulation (EC) No 1333/200811 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, ferrocyanides (E 535–538) are authorised food additives in the EU at 20 mg/kg in 2 categories listed in Table 2.
Table 2. MPLs of ferrocyanides (E 535–538) in foods according to the Annex II to Regulation (EC) No 1333/2008
阮等人(2012 年)研究了盐中添加不同浓度(2.5、7.5 和 100 mg/kg)的亚铁氰化钾 (K4[Fe(CN)6])对鳕鱼在盐渍、储存(长达 6 个月,在 ~ 2°C 下)和再水化过程中脂质氧化的影响。亚铁氰化物浓度的增加加速了盐渍鳕鱼的脂质氧化,如脂质氢过氧化物(PV)和TBARS的增加以及荧光化合物的形成所观察到的那样:在提取过程中的有机氯仿相 (δFOR)和甲醇水相(δFAQ)中测定了荧光偏移(δF)。盐渍鳕鱼的变黄(较高的 b* 值,b* 是黄度的指标)与较高水平的氧化衍生物有关。发现 PV、TBARS和游离脂肪酸(FFA)之间以及FFA 和 δFOR 之间具有高度相关性。主成分分析结果显示,TBARS、b*值和δFOR是脂质氧化的最强指标。
Dorazio 和 Bruckner(2015)提出了亚单原子亚氰化钠层对氯化钠晶体的作用方式的研究结果,通过成核抑制起到抗结剂的作用。由于在添加微黄色的三氯化铁(III)水溶液后形成普鲁士蓝(Fe4[Fe(CN)6]3·nH2O)后,会出现强烈的蓝绿色,因此很容易检测到商店购买的食盐上的亚铁氰化钠。
3.2 授权用途和使用水平
亚铁氰化物(E 535-538)的最高含量已在经修订的食品添加剂法规(EC)No 1333/200811 附件 II 中定义。在本文档中,这些级别称为最大使用量级别(MPL)。
目前,亚铁氰化物(E 535-538)是欧盟授权的食品添加剂,含量为 20 mg/kg,分为表 2 中列出的 2 类.
表 2.根据法规(EC)No 1333/2008 附件II的食品中亚铁氰化物 (E 535–538)的 MPL
Food category number | Food category name | E-number/group | Restrictions/exception | MPL (mg/L or mg/kg as appropriate) |
12.1.1 | Salt | E535–538a | 20b | |
12.1.2 | Salt substitutes | 535–538a | 20b |
MPL: maximum permitted level.
a The additives may be added individually or in combination.
b The maximum level is expressed as anhydrous potassium ferrocyanide.
Ferrocyanides (E535–538) are not authorised according to Annex III to Regulation (EC) No 1333/2008. (100 explanation: see (EC) No 1333_2008 EU food additives standard.pdf)
3.3 Exposure data
3.3.1 Reported use levels or data on analytical levels of ferrocyanides (E 535–538)
Most food additives in the EU are authorised at a specific MPL. However, a food additive may be used at a lower level than the MPL. Therefore, information on actual use levels is required for performing a more realistic exposure assessment.
In the framework of Regulation (EC) No 1333/2008 on food additives and of Commission Regulation (EU) No 257/2010 regarding the re-evaluation of approved food additives, EFSA issued a public call,12 for occurrence data (usage level and/or concentration data) on ferrocyanides (E 535–538). In response to this public call, updated information on the actual use levels of ferrocyanides (E 535–538) in foods was made available to EFSA by industry. No analytical data on the concentration of ferrocyanides (E 535–538) in foods were made available by the Member States.
Summarised data on reported use levels in foods provided by industry
Industry provided EFSA with data on use levels (n = 16) of ferrocyanides (E 535–538) in salt (FC 12.1.1) and in other foods containing salt (n = 62), and thus containing ferrocyanides (E 535–538),covering 11 food categories.
食品类别编号 | 食品类别名称 | 编号/组 | 限制/例外 | MPL(mg/L或mg/kg视情况而定) |
12.1.1 | 盐 | E535–538a | 20b | |
12.1.2 | 代盐制品 | 535–538a | 20b |
MPL:最大使用量
a 添加剂可以单独添加,也可以组合添加。
b 最高水平以无水亚铁氰化钾表示。
亚铁氰化物(E535–538)未根据法规(EC) No 1333/2008 的附件 III 获得授权。
(壹佰注:可参见 ![]() (EC) No 1333_2008 欧盟食品添加剂标准.pdf)
(EC) No 1333_2008 欧盟食品添加剂标准.pdf)
3.3 暴露数据
3.3.1 亚铁氰化物的报告使用水平或分析水平数据 (E 535–538)
欧盟的大多数食品添加剂都已获得特定 MPL 的授权。但是,食品添加剂的使用量可能低于 MPL。因此,需要有关实际使用水平的信息才能执行更真实的暴露评估。
在欧盟关于食品添加剂的法规(EC)No 1333/2008 和关于重新评估已批准食品添加剂的法规(EU)No 257/2010 的框架内,欧洲食品安全局EFSA 发出了公开呼吁,12 要求提供亚铁氰化物 (E 535-538) 的出现数据(使用水平和/或浓度数据)。为响应这一公众呼吁,各行业向 EFSA 提供了有关食品中亚铁氰化物 (E 535-538)实际使用量的最新信息。会员国没有提供关于食品中亚铁氰化物浓度(E 535-538)的分析数据。
按行业提供的食物中报告使用量汇总数据
工业界向 欧洲食品安全局EFSA 提供了盐(FC 12.1.1)和其他含盐食品(n = 62)中亚铁氰化物(E 535-538)使用量(n = 16)的数据,由此含有亚铁氰化物(E 535-538涵盖11个食品类别。
Updated information on the actual use levels of ferrocyanides (E 535–538) in foods was made available to EFSA by FoodDrinkEurope (FDE, Documentation provided to EFSA No. 3), European Potato Processors’ Association (EUPPA, Documentation provided to EFSA No. 4), European Salt Producers’ Association (EU_SALT, Documentation provided to EFSA No. 5), Ornua (Documentation provided to EFSA No. 6) and Intersnack (Documentation provided to EFSA No. 7).
Appendix A provides data on the use levels of ferrocyanides (E 535–538) in foods as reported by industry.
3.3.2 Summarised data extracted from the Mintel's Global New Products Database
The Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 2.5 million food and beverage products of which more than 900,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 20 out of its 28 member countries and Norway presented in the Mintel GNPD.
For the purpose of this Scientific Opinion, the Mintel's GNPD was used for checking the labelling of food and beverages products and food supplements for ferrocyanides (E 535–538) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to the Mintel's GNPD, ferrocyanides (E 535–538) were labelled on 399 products between January 2013 and April 2018.
Appendix B lists the percentage of the food products labelled with ferrocyanides (E 535–538) out of the total number of food products per food subcategories according to the Mintel's GNPD food classification. The percentages ranged from less than 0.1% in many food subcategories to 2.1% in the Mintel's GNPD food subcategory ‘Seasonings’ which includes products falling under legislation categories FCs 12.1.1 Salt and 12.1.2 Salt substitutes. Taking into account only the salt products from the sub-category ‘Seasonings’ (n = 1,533), 13% of them contained ferrocyanides (E 535–538).
All other subcategories presented in the Mintel's GNPD may contain ferrocyanides (E 535–538) as a carry-over from salt.
Considering the individual E numbers of ferrocyanides (E 535–538), the majority of the products were labelled with sodium ferrocyanide (E 535) (n = 305), whereas 101 products were labelled with potassium ferrocyanide (E 536). Some foods were labelled with a combination of both additives. No products were labelled with calcium ferrocyanide (E 538).
欧洲食品和饮料行业组织FoodDrinkEurope(FDE,文件提供给EFSA No.3)、欧洲马铃薯加工商协会(EUPPA,文件提供给EFSA No.4)、欧洲盐生产商协会(EU_SALT,文件提供给EFSA No.5)、Ornua(文件提供给EFSA No.6)和Intersnack(文件提供给EFSA No.7)向EFSA提供了有关食品中亚铁氰化物(E 535-538)实际使用水平的最新信息。
3.3.2 从英敏特(Mintel)全球新产品数据库中提取的汇总数据
英敏特的 GNPD 是一个在线数据库,用于监控全球市场上新推出的包装商品。它包含超过250万种食品和饮料产品的信息,其中超过 900,000 种已经或已经在欧洲食品市场上销售。英敏特于1996年开始覆盖欧盟食品市场,目前其28个成员国中有20个和挪威出现在英敏特 GNPD中。
就本科学意见而言,使用了英敏特的GNPD用于检查欧盟食品市场内食品和饮料产品以及食品补充剂的亚铁氰化物(E 535-538)标签,因为该数据库包含标签上的强制性成分信息。
根据英敏特的GNPD,2013年1月至2018年4月期间,399种产品上贴有亚铁氰化物(E 535-538)标签
附录B列出了根据Mintel的GNPD食品分类,贴有亚铁氰化物标签的食品商品 (E 535-538)占每个食品子类别的食品商品总数的百分比。百分比从许多食品子类别的不到0.1%到英敏特的GNPD食品子类别“调味料”的2.1%不等,其中包括属于FC 12.1.1盐和12.1.2代盐制品类别的产品。仅考虑来自“调味料”子类别的盐产品(n = 1,533),其中13%含有亚铁氰化物(E 535-538)。
英敏特的 GNPD 中列出的所有其他子类别可能含有亚铁氰化物 (E 535-538)作为盐的残留物。
考虑到亚铁氰化物的单个E编号(E 535-538),大多数产品标有亚铁氰化钠(E 535)(n = 305),而101种产品标有亚铁氰化钾(E 536)。一些食物被贴上了两种添加剂的组合标签。没有产品标示亚铁氰化钙(E 538)。
3.3.3 Salt intake data used for exposure assessment to ferrocyanides (E 535–538)
Ferrocyanides (E 535–538) are solely authorised in FCs 12.1.1 Salt and 12.1.2 Salt substitutes, it is therefore important to accurately assess the intake of salt to estimate their exposure. However, there are considerable challenges to accurately measure the usual salt intake in individuals (McLean et al., 2017). Dietary surveys are commonly not considered as a good source of information because a significant part of the salt intake is coming from the consumption of processed foods and their salt content is highly variable over time and food types so there is a high uncertainty using food composition tables.
Another issue is that people add salts to their food (e.g. during preparation or while eating). This part of the salt intake is not well covered in dietary surveys.
A typical way of estimation of the salt intake is its calculation from the urinary excretion of sodium. Urinary sodium excretion has traditionally been used as a biomarker of sodium intake (Gibson, 2005; Freedman et al., 2015), as it is considered to be more accurate than estimates of intake based on dietary assessments. Twenty-four-hour urinary sodium excretion is used as a measure of average sodium intake at the population level (WHO, 2011). In healthy people, almost all dietary sodium intake is absorbed. Urine is the major route of sodium excretion with mean recovery rates of dietary sodium in the urine generally ranging from 80% to 95%.
The ANS Panel decided to use estimated salt intake data from urinary excretion studies of sodium for the assessment of exposure to ferrocyanides (E 535–538) instead of the food consumption data from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) which are used in the other opinions related to the re-evaluation of food additives.
FC 12.1.2 salts substitutes which are not sodium-based (e.g. potassium chlorides) are only partly taken into account in the current estimates of ferrocyanides and this can lead to an underestimation.
The NDA Panel drafted an opinion13 on dietary reference values for sodium and submitted it to a public consultation in 2017. The draft opinion describes that in 2016 an overview of sodium intake in European populations was prepared based on data on sodium urinary excretion in European populations collected through EFSA focal points and the members of the EFSA Food Consumption Network. Data were received from 17 countries, and the most recent surveys, conducted between 2002 and 2014, were selected. Three countries provided urinary sodium excretion data in children (Austria, Iceland, Spain) and 16 countries provided urinary sodium excretion data in adults (Austria, Belgium, Croatia, the Czech republic, Finland, Germany, Greece, Hungary, Ireland, Norway, Slovenia, Spain, Sweden, Switzerland, the Netherlands and the United Kingdom).
3.3.3 用于亚铁氰化物暴露评估的盐摄入量数据(E 535-538)
亚铁氰化物(E 535-538)在 FCs 12.1.1 盐和 12.1.2 盐替代品中仅获得授权,因此准确评估盐的摄入量以估计其暴露量非常重要。然而,准确测量个体通常的盐摄入量存在相当大的挑战(麦克林等人,2017 年)。膳食调查通常不被认为是一个好的信息来源,因为盐摄入量的很大一部分来自加工食品的消费,而且它们的盐含量随时间和食物类型的变化很大,因此使用食物成分表存在高度不确定性。
另一个问题是人们在食物中添加盐(例如在准备或进食时)。这部分盐摄入量在膳食调查中没有得到很好的涵盖。
评估盐摄入量的一种典型方法是根据尿中钠的排泄量计算。尿钠排泄传统上被用作钠摄入量的生物标志物(Gibson,2005 年;Freedman 等人,2015 年),因为它被认为比基于膳食评估的摄入量估计更准确。24 小时尿钠排泄量用作衡量人群水平平均钠摄入量的指标(WHO,2011 年)。在健康人中,几乎所有的膳食钠摄入量都被吸收。尿液是钠排泄的主要途径,膳食钠在尿液中的平均回收率通常在 80% 至 95% 之间。
ANS 小组决定使用钠尿液排泄研究的估计盐摄入量数据来评估亚铁氰化物的暴露量(E 535-538),而不是来自EFSA 综合欧洲食品消费数据库(综合数据库)的食物消费数据,这些数据用于与食品添加剂重新评估相关的其他意见。
FC 12.1.2 非钠基盐替代品(例如氯化钾)在目前的亚铁氰化物估计中仅部分考虑,这可能导致低估。
NDA 小组起草了一份关于钠的膳食参考值的意见13,并于2017 年将其提交给公众咨询。意见草案描述,2016年,根据通过 EFSA 联络点和 EFSA 食品消费网络成员收集的欧洲人群钠尿排泄数据,准备了欧洲人群钠摄入量概述。收到了来自 17 个国家的数据,并选择了2002 年至 2014 年间进行的最新调查。3 个国家提供了儿童尿钠排泄数据(奥地利、冰岛、西班牙),16 个国家提供了成人尿钠排泄数据(奥地利、比利时、克罗地亚、捷克共和国、芬兰、德国、希腊、匈牙利、爱尔兰、挪威、斯洛文尼亚、西班牙、瑞典、瑞士、荷兰和英国)。
The majority of countries used 24-h urine collection, while three countries collected spot or timed urine collection and estimated daily sodium excretion through arithmetic extrapolation. Studies using 24-h urine collection were heterogeneous with respect to the methods and criteria applied for the assessment and exclusion of incomplete or unreliable urine collection (e.g. PABA recovery, creatinine excretion levels, urinary volume, self-reporting of incomplete samples). Some studies were designed as national monitoring surveys, while others were conducted as part of broader observational studies. Samples sizes also varied widely, from tens to thousands of people.
The NDA Panel noted that a single 24-h urine collection does not reliably reflect an individual's usual intake, primarily due to within person day-to-day variability in sodium intake and excretion. The Panel therefore considered that a single 24-h collection can be used to estimate average group sodium intakes, but can lead to random misclassification of study participants in relation to their usual sodium intake. In addition, the Panel noted that incomplete 24-h urine collections could have introduced bias in intake estimates.
More convenient methods such as casual spot and timed spot urine collections (i.e. collection during the day, evening, or overnight) have also been used as indicators of sodium intake. Day-to-day and diurnal variations in sodium excretion render these measures highly variable at the individual level; hence, these methods are subject to greater within-person variability in sodium excretion than 24-h urine collections (Ji et al., 2014; Wang et al., 2013; Sun et al., 2017). Predictive equations have been developed to estimate 24-h urinary sodium excretion from spot urine samples (Kawasaki et al., 1993; Tanaka et al., 2002; Brown et al., 2013).
The NDA Panel noted that both overnight and spot urine collections are easier for participants, but their reliability to estimate daily sodium intake is largely affected by circadian variations in individual sodium excretion. The NDA Panel further noted that estimates of individual daily intakes from predictive equations based on spot urine samples can be biased, particularly at the lower and higher ends of the distribution and are therefore unreliable.
The ANS Panel decided to take into account all surveys from the NDA opinion in the current assessment. Data on surveys and methodologies used for the estimation of salt intake and the exposure assessment of ferrocyanides (E 535–538) are presented in Appendix C. Sodium chloride intake (NaCl g/day (Y)) was calculated from the sodium excreted in urine (mmol Na/day (X)) reported in the publications with the following equation:
Y=[(X×22.99)/0.4]/1,000
Based on the fact that 22.99 g sodium equals to 1 mole of sodium, and 1 g of salt contains 0.4 g sodium and 0.6 g chloride.
大多数国家使用 24 小时尿液收集,而 3 个国家收集了点尿或定时尿液收集,并通过算术外推估计每日钠排泄量。使用 24 小时尿液采集的研究在用于评估和排除不完整或不可靠的尿液采集的方法和标准方面存在异质性(例如 PABA 恢复、肌酐排泄水平、尿量、不完整样本的自我报告)。一些研究被设计为全国监测调查,而另一些研究则作为更广泛的观察性研究的一部分进行。样本量也差异很大,从几十人到几千人不等。
NDA 小组指出,单次 24 小时尿液收集并不能可靠地反映个体的正常摄入量,主要是由于人体内钠摄入量和排泄量的日常变化。因此,小组认为,单个 24 小时集合可用于估计平均组钠摄入量,但可能导致研究参与者根据其通常的钠摄入量进行随机错误分类。此外,评估小组指出,不完整的 24 小时尿液收集可能会在摄入量估计中引入偏倚。
更方便的方法,如随意点尿和定时点尿采集(即白天、晚上或过夜采集)也被用作钠摄入量的指标。钠排泄的日常和昼夜变化使这些指标在个体水平上具有很大差异;因此与 24 小时尿液收集相比,这些方法在钠排泄方面受到更大的人内变异性的影响(Ji等人,2014 年;Wang et al.,2013;Sun等人,2017 年)。已经开发了预测方程来估计随机尿样中 24 小时尿钠排泄量(Kawasaki 等人,1993 年;Tanaka et al., 2002;Brown et al.,2013)。
NDA 小组指出,过夜和随机尿液收集对参与者来说都更容易,但他们估计每日钠摄入量的可靠性在很大程度上受到个体钠排泄的昼夜节律变化的影响。NDA小组进一步指出,根据随机尿液样本的预测方程对个人每日摄入量的估计可能存在偏差,尤其是在分布的低端和高端,因此不可靠。
ANS 小组决定在当前评估中考虑来自 NDA 意见的所有调查。附录 C 中提供了用于估计盐摄入量和亚铁氰化物暴露评估(E 535-538)的调查和方法数据。氯化钠摄入量(NaCl g/天(Y))是根据出版物中报告的尿液中排泄的钠(mmol Na/天(X))计算得出的,公式如下:
Y=[(X×22.99)/0.4]/1,000
基于22.99g钠等于1 mol钠,1g克盐含有0.4 g钠和0.6 g氯化物。
3.4 Exposure estimate
3.4.1 Exposure to ferrocyanides (E 535–538) from their use as food additives
The Panel estimated chronic exposure to ferrocyanides (E 535–538) for children and adolescents (boys and girls at the age of 6–18), adults and the elderly (men and women at the age of 18–79) for different Member States. Dietary exposure to ferrocyanides (E 535–538) was calculated by multiplying ferrocyanides (E 535–538) concentrations with all estimated salt consumption amounts reported in Appendix C, including the mean and high (up to the 75th percentile) consumption. Exposure estimates per kg body weight were obtained by using the standard body weight for each age group (EFSA, 2012).
Exposure to ferrocyanides (E 535–538) was estimated by the ANS Panel based on two different sets of concentration data: (1) MPL as set down for FC 12.1.1 Salt in the EU legislation (defined as the regulatory maximum level exposure assessment scenario); and (2) mean use level reported by industry for FC 12.1.1. Salt of 9.7 mg/kg (defined as the refined exposure assessment scenario). The Panel noted that the highest reported use level of ferrocyanides (E 535–538) in salt was equal to the MPL.
Dietary exposure to ferrocyanides (E 535–538)
Table 3 summarises the estimated exposure to ferrocyanides (E 535–538) from their use as food additives in the population groups according to the different exposure scenarios. Detailed results per population group and survey are presented in Appendix D.
Dietary exposure to ferrocyanides was calculated based on mean and high levels consumption of salts.
Table 3. Summary of dietary exposure to ferrocyanides (E 535–538) from their use as food additives in the maximum level exposure assessment scenario and in the refined exposure scenario, mg anhydrous potassium ferrocyanide/kg bw per day.
Children and adolescents (6–18 years) | Adults and elderly (18–79 years) | |||
Min | Max | Min | Max | |
MPL scenario | ||||
Mean | 0.003 | 0.007 | 0.002 | 0.004 |
Higha | 0.004 | 0.009 | 0.002 | 0.005 |
Refined scenario | ||||
Mean | 0.002 | 0.004 | 0.001 | 0.002 |
Higha | 0.002 | 0.004 | 0.001 | 0.003 |
a High levels up to p75 (see Appendix C).
3.4 暴露量估计
3.4.1 亚铁氰化物 (E 535–538) 用作食品添加剂的暴露量
该小组估计了不同会员国的儿童和青少年(6-18 岁的男孩和女孩)、成人和老年人(18-79 岁的男性和女性)长期接触亚铁氰化物 (E 535-538)。膳食中亚铁氰化物 (E 535-538) 的摄入量是通过将亚铁氰化物 (E 535-538) 浓度乘以附录 C 中报告的所有估计盐消费量来计算的,包括平均和高(最高第 75 个百分位数)摄入量。通过使用每个年龄组的标准体重获得每公斤体重的暴露估计值 (EFSA, 2012)。
亚铁氰化物暴露量 (E 535-538) 由 ANS 小组根据两组不同的浓度数据进行估计:(1) 欧盟立法中为 FC 12.1.1 盐设定的 MPL(定义为监管最高水平暴露评估情景);(2) FC 12.1.1 按行业报告的平均使用水平。盐含量为 9.7 mg/kg(定义为精制盐接触评估情景)。评估小组指出,盐中亚铁氰化物的最高报告使用量 (E 535-538) 与 MPL 相当。
膳食中亚铁氰化物的暴露(E 535–538)
表 3 总结了根据不同的接触情景,人群因使用亚铁氰化物(E 535-538)作为食品添加剂而对亚铁氰化物(E 535-538)的估计暴露量。附录 D 中介绍了每个人群和调查的详细结果。
从膳食摄入亚铁氰化物的分量是根据盐的平均摄入量和高水平计算的。
表 3.在最高摄入量评估情景和精制盐接触情景中,因将亚铁氰化物用作食品添加剂而从膳食摄入亚铁氰化物 (E 535–538) 的摘要,无水亚铁氰化钾毫克/千克体重/每天
儿童和青少年 (6-18 岁) | 成人和老年人 (18-79 岁) | |||
最小 | 最大限度 | 最小 | 最大限度 | |
MPL情景 | ||||
平均值 | 0.003 | 0.007 | 0.002 | 0.004 |
最大值a | 0.004 | 0.009 | 0.002 | 0.005 |
精制盐情景 | ||||
平均值 | 0.002 | 0.004 | 0.001 | 0.002 |
最大值a | 0.002 | 0.004 | 0.001 | 0.003 |
a 高达 p75 的高水平(见附录 C)。
In the regulatory maximum level exposure assessment scenario, based on the mean consumption of salt, the exposure to ferrocyanides (E 535–538) from their use as a food additive ranged from 0.002 mg/kg bw per day in adults and elderly to 0.007 mg/kg bw per day in children and adolescents. For the high consumers of salt, exposure in the same scenario ranged from 0.002 mg/kg bw per day in adults and elderly to 0.009 mg/kg bw per day in children and adolescents.
In the refined estimated exposure scenario, based on with the mean consumption of salt the exposure ranged from 0.001 mg/kg bw per day in adults and elderly to 0.004 mg/kg bw per day in children and adolescents. For the high consumers of salt, exposure was in the same ranges.
Uncertainty analysis
Uncertainties in the exposure assessment of ferrocyanides (E 535–538) have been discussed above. In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), the following sources of uncertainties have been considered and summarised in Table 4.
Table 4. Qualitative evaluation of influence of uncertainties on the dietary exposure estimate
Sources of uncertainties | Directiona |
Use of urinary excretion studies for the assessment of salt intake Sodium intake data: different methodologies/representativeness Sodium intake does not only originate from salt Use of standard body weights for the exposure assessment Assumption that all salt contains the additives while the Mintel's GNPD indicates that only 13% of the salt products in the database contain ferrocyanides (E 535 and E 536) Salt substitutes partly considered in the exposure assessment (only their sodium content is taken into account) Regulatory maximum level exposure assessment scenario: .exposure calculations based on the MPL according to Annex II to Regulation (EC) No 1333/2008 Refined exposure assessment scenarios: .exposure calculations based on the mean levels (reported use from industries) Assumption that reported use levels were expressed as anhydrous ferrocyanide salts | +/– +/– + +/– +
–
+
+/–
+ |
a + ,uncertainty with potential to cause overestimation of exposure; –, uncertainty with potential to cause underestimation of exposure.
在监管最高水平暴露评估情景中,根据盐的平均消费量,亚铁氰化物 (E 535-538)作为食品添加剂的暴露量从成人和老年人每天 0.002 毫克/公斤体重到儿童和青少年每天 0.007 毫克/公斤体重不等。对于盐摄入量高的人群,在相同情况下,成人和老年人每天的摄入量为每公斤体重0.002毫克,儿童和青少年每天的摄入量为每公斤体重0.009毫克。
在精制盐评计接触量情景中,根据盐的平均摄入量,接触量从成人和老年人每天每公斤体重0.001毫克到儿童和青少年每天每公斤体重0.004毫克不等。对于盐的高消费量,暴露量在相同的范围内。
不确定性分析
上面已经讨论了亚铁氰化物(E 535–538)暴露评估中的不确定性。根据欧洲食品安全局(EFSA)关于膳食暴露评估中不确定性的意见(EFSA,2007)中提供的指导,考虑并总结了以下不确定性的来源,见表4。
表 4.不确定性对膳食暴露估计影响的定性评价
不确定性的来源 | 方向a |
使用尿液排泄研究来评估盐摄入量 | +/– |
钠摄入量数据:不同的方法/代表性 | +/– |
钠摄入量不仅来自盐 | + |
使用标准体重进行暴露评估 | +/– |
假设所有盐都含有添加剂,而Mintel 的GNP表明数据库中只有 13%的盐产品含有亚铁氰化物(E535 - E536) | + |
暴露评估中部分考虑的盐替代品(仅其钠含量被考虑在内) | – |
监管最高水平暴露评估情景: | |
根据法规(EC)No 1333/2008附件II的MPL计算 | + |
精制盐的暴露评估情景: | |
基于平均水平的暴露计算(报告的工业使用量) | +/– |
假设报告的使用量为无水亚铁氰酸盐表示 | + |
a +,可能导致高估暴露的不确定性;–,不确定性可能导致低估暴露。
Overall, the Panel considered that the uncertainties identified indicate an overestimation of the exposure to ferrocyanides (E 535–538) as food additives in European countries for both the regulatory maximum level and the refined exposure scenario.
3.5 Biological and Toxicological data
No toxicological information was submitted for the re-evaluation of sodium, potassium and calcium ferrocyanide following an EFSA public call for data, prior to the start of this re-evaluation.
3.5.1 Absorption, distribution, metabolism and excretion
The relevant studies evaluated by JECFA (1974a, 1975), as well as the additional studies are summarised below.
Rats were dosed orally with 200 mg/kg bw potassium ferrocyanide. Approximately 47% was reported to have been excreted unchanged in the faeces and 3% in the urine. Faecal and urinary excretion of ferrocyanide and thiocyanate was at a maximum from day 1 to 3 after dosing and thereafter declined to a low level (unpublished data from Gage (1950), cited in JECFA (1975).
Female Wistar rats (250–280 g, 3 animals) were administered 59Fe- and 14C dual-labelled potassium ferrocyanide as K4[59Fe(14CN)6]·3H2O in a single dose of 10 mg per animal by gastric intubation (Nielsen et al., 1990a). Urine and faeces were collected for 7 days following administration. 14C was measured in expired air during the first 24 h after administration. Whole body retention (WBR) of 59Fe was measured after 7 days and 59Fe and 14C was measured in blood, liver, spleen, kidneys, heart/lung, gut and carcass after 7–10 days. Total 59Fe-activity (mean ± SD) in faeces and urine after 7 days was 94.4 ± 2.9% and 2.5 ± 0.8% of dose, respectively. The total absorption of potassium ferrocyanide was calculated by the authors to be up to 5.6% (based on subtracting percentage 59Fe-activity in faeces from 100% of dose). WBR and total recovery of 59Fe (mean ± SD) was 0.09 ± 2.1 and 97 ± 2.1% of the dose, respectively. The erythrocyte incorporation (mean ± SD) of 59Fe was 0.005 ± 0.0007% of the dose. Total 14C-activity (mean ± SD) in urine after 7 days was 2.8 ± 0.5% of dose. 59Fe-activity was detected in liver, spleen, kidney and heart/lungs. The amount of expired 14C (mean ± SD) during 24 h after gastric intubation was 0.04 ± 0.01% of administered dose. The authors estimated that less than 0.06 mg free cyanide/kg bw was absorbed after oral administration of 10 mg 59Fe- and 14C-labelled potassium ferrocyanide.
Likewise, following an oral dose of 500 mg (6.2–7.1 mg/kg-bw) of potassium ferric ferrocyanide in humans (n = 3, male), absorption was 0.25%–0.42% of the FeII and FeIII, respectively, and whole body retention after seven days was 0.03–0.07% (Nielsen et al., 1990b). Elimination was > 97% and > 99% faecal in rats and humans, respectively.
总体而言,评估小组认为,所确定的不确定性表明,在监管最高水平和精制盐接触情景中,欧洲国家高估了作为食品添加剂的亚铁氰化物 (E 535-538) 的暴露量。
3.5 生物和毒理学数据
在 EFSA 公开征集数据后,在重新评估之前,没有提交毒理学信息用于重新评估亚铁氰化物钠、钾和钙。
3.5.1 吸收、分布、代谢和排泄
JECFA (1974a, 1975) 评估的相关研究以及其他研究总结如下。
大鼠经口给予 200 mg/kg bw 亚铁氰化钾。据报道,大约 47% 的粪便排泄量保持不变,3% 从尿液中排泄。给药后第 1 天至第 3 天,亚铁氰化物和硫氰酸盐的粪便和尿液排泄量达到最大值,此后下降到低水平(Gage (1950) 未发表的数据,引自 JECFA (1975)。
雌性 Wistar 大鼠(250-280 g,3 只动物)通过胃插管以每只动物 10 mg 的单剂量给予 59Fe 和 14C 双标记亚铁氰化钾 K4[59Fe(14CN)6]·3H2O(Nielsen et al., 1990a)。给药后 7 天收集尿液和粪便。在给药后的前 24 小时内在呼出空气中测量 14C。7 天后测量 59Fe 的全身滞留 (WBR),7-10 天后测量血液、肝脏、脾脏、肾脏、心脏/肺、肠道和胴体的 59Fe 和 14C。7 天后粪便和尿液中总 59Fe 活性 (平均 ± SD) 分别为剂量的 94.4 ± 2.9% 和 2.5 ± 0.8%。作者计算出亚铁氰化钾的总吸收率高达 5.6%(基于 100% 剂量减去粪便中 59Fe 活性的百分比)。WBR 和 59Fe 的总恢复率(平均值 ± SD)分别为 0.09 ± 2.1 和 97 ± 2.1% 的剂量。59Fe 的红细胞掺入 (平均 ± SD) 为剂量的 0.005 ± 0.0007%。7 天后尿液中的总 14C 活性 (平均 ± SD) 为剂量的 2.8 ± 0.5%。在肝、脾、肾和心/肺中检测到 59 Fe 活性。胃插管后 24 小时内呼出的 14C (平均值 ± SD) 的量为给药剂量的 0.04 ± 0.01%。作者估计,口服 10 毫克 59 Fe 和 14C 标记的亚铁氰化钾后,每 kg 体重吸收的游离氰化物少于 0.06 毫克。
同样,在人类(n = 3,男性)口服 500 毫克(6.2-7.1 毫克/千克-体重)的亚铁氰化铁钾后,FeII 和 FeIII 的吸收率分别为 0.25%-0.42%,7 天后全身滞留率为 0.03-0.07%(Nielsen 等人,1990b)。大鼠和人类粪便>的消除率分别为97% 和 > 99%。
Overall, potassium ferrocyanide was absorbed to a limited extent from the gastrointestinal tract following oral administration to rats (unpublished data from Gage (1950), cited in JECFA (1975); Nielsen et al., 1990a). The majority was excreted unchanged in the faeces (approximately 95% of the dose). Ferrocyanide was detected in liver, spleen, kidney, heart and lungs. Free iron was detected in erythrocytes and free cyanide was detected in urine and expired air. The exposure to free cyanide was estimated to be less than 0.06 mg/kg bw after oral administration to rats of 10 mg 59Fe- and 14C-labelled potassium ferrocyanide per animal (Nielsen et al., 1990a). In humans, absorption was 0.25–0.42% (Nielsen et al., 1990b).
3.5.2 Acute toxicity
The oral LD50 value for potassium ferrocyanide was reported to be between 1600 and 3,200 mg/kg bw in rats (unpublished data by Fassett cited in JECFA (1975)).
3.5.3 Short-term and subchronic toxicity
Rats
Rats (10 animals/sex per group) were given 0%, 0.05%, 0.5% or 5% sodium ferrocyanide in the diet for 13 weeks (equivalent to 0, 45, 450 and 4,500 mg/kg bw per day using the EFSA default value of 0.09 for subchronic studies (EFSA, 2012)) (unpublished study by Oser (1959), cited in JECFA (1975)) Slightly lower growth rate and food consumption was observed at the 5% level. Haematocrit and haemoglobin values were lower at the 5% level. Kidney weight was higher at the 5% level (both sexes) and at the 0.5% level (females). Male adrenal and female pituitary gland weights were higher at the 5% level. A minimal degree of tubular damage in the kidneys was observed in rats at the 0.5% level. The effect was more marked at the 5% level where granular and calcified deposits were observed The Panel noted that the ADI set by JECFA for sodium, potassium and calcium ferrocyanide was based on this study. No further details included in the JECFA evaluation.
Dogs
Beagles (4 animals/sex per group) were given 0, 10, 100 or 1,000 mg/kg sodium ferrocyanide in the diet (equivalent to 0, 0.25, 2.5 and 25 mg/kg bw per day) for 13 weeks (unpublished data from Morgaridge (1970), cited in JECFA (1975)). No treatment-related effects were observed in appearance, behaviour, body weight change, physical condition, urinary pathology, haematology, biochemical parameters, or gross and histopathology.
Overall, treatment-related effects were observed in kidneys (higher organ weight, tubular damage and granular and calcified deposits) in rats given 0.5% and 5% sodium ferrocyanide (450 and 4,500 mg/kg bw per day) in the diet for 13 weeks (unpublished study by Oser (1959), cited in JECFA (1975)). No treatment-related effects were observed in dogs given dietary sodium ferrocyanide up to 1,000 mg/kg (25 mg/kg bw per day) for 13 weeks (Unpublished data from Morgaridge (1970), cited in JECFA (1975).
总体而言,大鼠口服给药后,亚铁氰化钾在有限程度上从胃肠道吸收(来自 Gage (1950) 的未发表数据,引自 JECFA (1975);Nielsen et al., 1990a)。大多数在粪便中排泄不变 (约占剂量的 95%)。在肝脏、脾脏、肾脏、心脏和肺中检测到亚铁氰化物。在红细胞中检测到游离铁,在尿液和呼出的空气中检测到游离氰化物。每只动物口服10毫克59铁和14C标记的亚铁氰化钾后,估计摄入游离氰化物的分量低于每公斤体重0.06毫克(Nielsen等人,1990a)。在人类中,吸收率为 0.25-0.42%(Nielsen 等人,1990b)。
3.5.2 急性毒性
据报道,大鼠口服亚铁氰化钾的LD50值介乎每公斤体重1600至3,200毫克之间(Fassett的未发表数据,引自JECFA (1975))。
3.5.3 短期和亚慢性毒性
大鼠
大鼠(每组 10 只动物/性别)在饮食中给予 0%、0.05%、0.5% 或 5% 亚铁氰化钠 13 周(相当于每天 0、45、450 和 4,500 毫克/千克体重,使用 EFSA 亚慢性研究的默认值 0.09(EFSA,2012 年))(Oser (1959)未发表的研究,引自 JECFA (1975))在 5% 的水平上观察到生长速率和食物消耗略低。血细胞比容和血红蛋白值低于 5% 水平。肾脏重量在 5% 水平 (雌雄) 和 0.5% 水平 (雌性) 较高。雄性肾上腺和女性垂体重量较高,为 5% 水平。在 0.5% 水平的大鼠中观察到肾脏肾小管损伤的最小程度。在观察到颗粒状和钙化沉积物的 5% 水平上,效果更为明显。该小组指出,JECFA 为钠、钾和亚铁氰化钙设定的每日允许摄入量是基于这项研究。JECFA 评估中不包括更多详细信息。
狗
比格犬(每组 4 只动物/性别)在饮食中给予 0、10、100 或 1,000 毫克/千克亚铁氰化钠(相当于每天 0、0.25、2.5 和 25 毫克/千克体重),持续 13 周(来自 Morgaridge (1970) 的未发表数据,引自 JECFA (1975))。在外观、行为、体重变化、身体状况、泌尿系统病理学、血液学、生化参数或大体和组织病理学方面未观察到与治疗相关的影响。
总体而言,在饮食中加入0.5%和5%亚铁氰化钠(每天每公斤体重450至4,500毫克)13周的大鼠的肾脏(器官重量增加、肾小管损伤以及颗粒状和钙化沉积物)观察到治疗相关效果(Oser(1959)未发表的研究,引自JECFA(1975))。在饮食中给予高达 1,000 mg/kg(每天 25 mg/kg 体重)的狗 13 周,未观察到与治疗相关的影响(来自 Morgaridge (1970) 的未发表数据,引自 JECFA (1975)。
3.5.4 Genotoxicity
No data were submitted to EFSA following a public call for data. Additional data were identified in the literature search and are summarised below. Neither the SCF (1991) nor JECFA (1970a, 1974a, 1975) have described any data on genotoxicity of sodium, potassium or calcium ferrocyanide.
Potassium ferrocyanide was reported to be negative at a concentration of 2.5 mM when tested for mutagenicity in a Rec-assay system with Bacillus subtilis strains H17 and M45 (Nishioka, 1975).
Potassium ferrocyanide was reported to be negative at concentrations ranging from 5 to 500 mM when tested for mutagenicity in a Rec-assay system with B. subtilis strains H17 and M45 (Kanematsu et al., 1980).
Potassium ferrocyanide was reported to be negative at concentrations ranging from 1 to 10,000 nM/mL when tested for genotoxic potential in the SOS Chromotest using Escherichia coli strains PQ37 and PQ35 with or without metabolic activation (Olivier and Marzin, 1987).
Sodium and potassium ferrocyanide were tested for genotoxicity in human lymphocyte cells in an in vitro Comet assay (Basu et al., 2013). The compounds were tested at concentrations of 0, 1, 5 and 10 mM for 3 h. The tail DNA (%) (± SEM) was increased after treatment with potassium ferrocyanide at 5 mM (3.55 ± 0.12) and 10 mM (4.22 ± 0.5) mM when compared to control (2.16 ± 0.15) (p ≤ 0.05). Sodium ferrocyanide did not induce DNA damage. Potassium ferrocyanide significantly reduced cell viability at all tested concentrations for about 20% compared to control, whereas sodium ferrocyanide significantly reduced cell viability for approximately 17% at the highest tested concentration only (10 mM).
In summary, potassium ferrocyanide did not show a mutagenic potential in two Rec assays with B. subtilis strains H17 and M45 in concentrations up to 500 mM (Nishioka, 1975, Kanematsu et al., 1980) or a genotoxic potential in the SOS Chromotest using E. coli strains PQ37 and PQ35 at in concentrations up to 10 mM Olivier and Marzin, 1987). Increased DNA damage was reported in an in vitro indicator assay with potassium but not sodium ferrocyanide at high doses. The Panel noted that the effect may be related to an indirect mechanism, such as ROS generation under in vitro conditions, which is based on the evidence that in food systems potassium ferrocyanide promotes lipid oxidation (Hansen et al., 1996; Nguyen et al., 2012).
Overall, the Panel considered that the use of ferrocyanides as food additives is not of genotoxic concern.
3.5.5 Chronic toxicity and carcinogenicity
Neither the SCF (1991) nor JECFA (1970a, 1974a, 1975) have described any data on chronic toxicity and carcinogenicity of sodium, potassium or calcium ferrocyanide.
3.5.4 遗传毒性
在公开征集数据后,没有数据提交给 EFSA。在文献检索中确定了其他数据,总结如下。SCF(1991)和 JECFA(1970a,1974a,1975)均未描述任何关于钠、钾或亚铁氰化钙遗传毒性的数据。
据报道,在枯草芽孢杆菌菌株H17 和 M45 的 Rec 测定系统中进行致突变性测试时,亚铁氰化钾浓度为2.5 mM(Nishioka,1975)。
据报道,在枯草芽孢杆菌菌株 H17 和 M45 的 Rec 测定系统中测试致突变性时,亚铁氰化钾在5 至 500 mM 的浓度范围内为阴性(Kanematsu et al.,1980)。
据报道,当使用大肠杆菌菌株 PQ37 和 PQ35 在 SOS Chromotest 中测试遗传毒性潜力时,亚铁氰化钾在浓度范围为 1 至 10,000 nM/mL 时呈阴性,有或没有代谢激活(Olivier 和 Marzin,1987)。
在体外彗星测定中测试了亚铁氰化钠和亚铁氰化钾在人淋巴细胞中的遗传毒性(Basu等人,2013 年)。在 0、 1 、5 和 10 mM 的浓度下对化合物进行 3 小时的测试。与对照组(2.16 ± 0.15)相比,用 5 mM(3.55 ± 0.12)和 10 mM(4.22 ± 0.5)mM 处理后,尾部 DNA (%)(± SEM)增加(p ≤ 0.05)。亚铁氰化钠不会诱导 DNA 损伤。与对照相比,亚铁氰化钾在所有测试浓度下显著降低了约20% 的细胞活力,而亚铁氰化钠仅在最高测试浓度(10 mM)下显著降低了约 17% 的细胞活力。
总之,在对浓度高达 500 mM 的枯草芽孢杆菌菌株 H17 和 M45 的两次 Rec 测定中,亚铁氰化钾未显示出诱变潜力(Nishioka,1975,Kanematsu 等人,1980),或使用浓度高达 10 mM Olivier 和 Marzin 的大肠杆菌菌株 PQ37 和 PQ35 的 SOS 染色体测试中未显示出遗传毒性潜力, 1987)。 在高剂量下用钾而不是亚铁氰化钠的体外指示剂测定中报告了 DNA 损伤增加。评估小组指出,这种影响可能与间接机制有关,例如在体外条件下产生ROS,这是基于在食品系统中亚铁氰化钾促进脂质氧化的证据(Hansen等,1996;Nguyen et al.,2012)。
整体而言,评估小组认为使用亚铁氰化物作为食物添加剂不构成基因毒性问题。
3.5.5 慢性毒性和致癌性
SCF(1991)和 JECFA (1970a, 1974a, 1975) 都没有描述任何关于钠、钾或亚铁氰化钙的慢性毒性和致癌性的数据。
In a study carried out at BIBRA (British Industrial Biological Research Association) between 1974 and 1976, Wistar rats (48 animals/sex per group, initial body weight 40–60 g) were given 0, 50, 500 or 5,000 mg/kg sodium ferrocyanide decahydrate in the diet (equal to 0, 4.4, 45 and 450.7 mg/kg bw per day for males and 0, 6.2, 62.5 and 630.1 mg/kg bw per day for females) for 2 years (COT, 1994b). The Panel noted that no analyses were carried out to verify the concentrations of ferrocyanide in the various diets. The Panel further noted that it was a pre-GLP study, but that the BIBRA GLP Unit audited the study to ensure that the results accurately reflects the raw data generated during the study. There are some further inadequacies in the 2-year study as compared by current standards. No clinical biochemistry parameters were measured and several organs were missing for histopathological examination. However, The Panel considered none of the inadequacies large enough to invalidate the study.
Rats were observed on a daily basis for abnormalities of appearance and behaviour or signs of ill-health and weighed regularly throughout the study (first day of treatment; first week; every fortnight during the first year and every month for the remainder of the study). Food and water intake were recorded approximately once a fortnight (first year) and at monthly intervals (second year). Blood was sampled during weeks 14, 26 and 54 from 12 rats/sex from the 0, 500 and 5,000 mg/kg groups and from all surviving rats sacrificed at the end of the study. Blood was analysed for total red blood cell (RBC) and white blood cell (WBC) counts, differential WBC count (0 and 5,000 mg/kg groups only), haemoglobin concentration, packed cell volume (PCV) and reticulocyte count (0 and 5,000 mg/kg groups only). Urine from 12 rats/sex (0 and 5,000 mg/kg groups) and 10 rats/sex (5 and 500 mg/kg groups) was sampled at 2 months intervals until week 104 were urine from all surviving animals was collected. Urine collected over a 6-h period was analysed for volume, specific gravity, pH, glucose, blood, bilirubin, ketones and protein. Urine collected over a 2-h period immediately following an oral water load of 25 mL/kg was analysed for volume, specific gravity and cell content. Urine collected over a 6-h period commencing 18 h after an oral water load of 25 mL/kg was analysed for volume and specific gravity. Animals that died during the study and all animals sacrificed at the end of the study were subjected to a post mortem examination. Major organs were weighed and adrenal, aorta, bladder, brain, caecum, colon, duodenum, eye, Harderian gland, heart, ileum, kidney, liver, lung, lymph node, mammary, muscle, nerve, oesophagus, ovary, pancreas, pituitary, prostate, rectum, salivary, seminal vesicles, spinal cord, spleen, stomach, testis, thymus, thyroid, trachea, uterus and vagina were investigated for non-neoplastic and neoplastic findings. The average daily intakes of sodium ferrocyanide were estimated to be equal to 0, 4.4, 45 or 450.7 mg/kg bw per day for males and 0, 6.2, 62.5 or 630.1 mg/kg bw per day for females given 0, 50, 500 or 5,000 mg/kg sodium ferrocyanide in the diet.
在 1974 年至 1976 年间在 BIBRA(英国工业生物研究协会)进行的一项研究中,Wistar 大鼠(每组 48 只动物/性别,初始体重 40-60 克)在饮食中给予 0、50、500 或 5,000 毫克/千克亚铁氰化钠十水合物(相当于雄性每天 0、4.4、45 和 450.7 毫克/千克体重,0, 女性每天每公斤体重 6.2、62.5 和 630.1 毫克)(COT,1994b)。评估小组指出,没有进行分析以验证各种饮食中亚铁氰化物的浓度。评估小组进一步指出,这是一项 GLP 前研究,但 BIBRA GLP 部门对该研究进行了审计,以确保结果准确反映了研究期间生成的原始数据。与当前标准相比,为期 2 年的研究还存在一些进一步的不足。未测量临床生化参数,组织病理学检查缺失几个器官。然而,该小组认为没有任何不足之处大到足以使该研究无效。
每天观察大鼠的外观和行为异常或健康状况不佳的迹象,并在整个研究过程中定期称重 (治疗的第一天;第一周;第一年每两周一次,研究的其余部分每月称重)。大约每两周(第一年)和每月(第二年)记录一次食物和水的摄入量。在第 14 周、第 26 周和第 54 周,从 0、500 和 5,000 mg/kg 组的 12 只大鼠/性别以及研究结束时处死的所有存活大鼠中取血。分析血液中的总红细胞(RBC)和白细胞(WBC)计数、分类 WBC 计数(仅限 0 和 5,000 mg/kg 组)、血红蛋白浓度、压滤细胞体积(PCV)和网织红细胞计数(仅限 0 和 5,000 mg/kg 组)。每隔 2 个月取样 12 只大鼠/性别(0 和 5,000 mg/kg 组)和 10 只大鼠/性别 (5 和 500 mg/kg 组) 的尿液,直到第 104 周收集所有存活动物的尿液。分析在 6 小时内收集的尿液的体积、比重、pH 值、葡萄糖、血液、胆红素、酮体和蛋白质。分析在口服水负荷为 25 zmlL/kg 后立即在 2 小时内收集的尿液的体积、比重和细胞含量。分析口服水负荷为25ml/kg 后18小时开始的6小时内收集的尿液的体积和比重。在研究期间死亡的动物和在研究结束时处死的所有动物都要进行尸检。称重主要器官,检查肾上腺、主动脉、膀胱、脑、盲肠、结肠、十二指肠、眼、哈德腺、心脏、回肠、肾脏、肝脏、肺、淋巴结、乳腺、肌肉、神经、食道、卵巢、胰腺、垂体、前列腺、直肠、唾液、精囊、脊髓、脾脏、胃、睾丸、胸腺、甲状腺、气管、子宫和阴道的非肿瘤和肿瘤发现。估计雄性每天平均摄入每公斤体重0、4.4、45或450.7毫克,而雌性每天摄入0、50、500或5,000毫克亚铁氰化钠的摄入量则为每公斤体重0、6.2、62.5或630.1毫克
Rats (both sexes) from the highest dose group drank more water compared to the controls and the other two dose groups during part of the first 9 months of the study. Except for a higher number of cells excreted in 2-h urine samples from treated animals (all levels of treatment but most frequently in the mid- and high-dose groups and not consistently throughout the study) compared with controls (p < 0.05 to p < 0.001), no treatment-related adverse effects were observed in the urine analysis. There were higher incidences of pneumonia in male rats from the 5,000 mg/kg group (20/48 animals, p < 0.01) and of emphysema in both the 500 and 5,000 mg/kg groups (7/48 animals, p < 0.01 and 13/48 animals, p < 0.001, respectively) compared to control. No other statistically significant treatment-related effects were reported (COT, 1994b).
In a study performed concurrently with the 2-year study, rats (12 animals/sex per group) were given 0, 50, 500 or 5,000 mg/kg sodium ferrocyanide decahydrate in the diet for 49 weeks (COT, 1994b). Blood was sampled at the end of the study and analysed for total RBC and WBC counts, differential WBC count, haemoglobin concentration, PCV and reticulocyte count. Urine samples were taken at weeks 47–49 from all rats and analysed for bilirubin, glucose, blood, ketones and protein. In addition renal function tests were performed. Full post-mortems and histopathological examination of heart, kidney, liver, lung and spleen was carried out on all rats. There was a statistically significant increase in the mean number of cells excreted per hour in 2-h urine samples of treated animals (both sexes) from the 500 and 5,000 mg/kg groups compared with controls (males: 683, 533, 1,517 and 4,000; females 250, 418, 4,200 and 4,782 in the 0, 50, 500 and 5,000 mg/kg groups, respectively). In addition, the concentrations of urine samples from both sexes in the highest treatment group taken at 6 and 18 h were statistically significantly higher compared to controls.
Overall, no carcinogenic effect was seen in these studies and neither were there any non-neoplastic findings observed considered to be of toxicological relevance. In particular, no treatment-related effects were observed in kidneys of rats given 0, 4.5, 45 or 450 mg sodium ferrocyanide/kg bw per day, for either 49 weeks or 2 years (COT, 1994b). However, in the 2-year study, mid- and high dose animals frequently showed a higher cell excretion rate in 2-h urine samples than did controls. This effect was seen in both males and females, although it was not consistent and inter-animal variation in the parameter was frequently large. An increased cell excretion rate compared to controls was also seen at the low-dose group but only on three occasions. No adverse renal effects were seen at histopathological examination, other than a slight increase in incidence and severity of glomerulonephrosis in males in the first interim study. Nevertheless, since the kidneys are known to be the target organ for ferrocyanide toxicity, the Panel considered the increased cell excretion rate indicative for occasional, transient kidney toxicity and a NOAEL of 50 mg/kg diet (equal to an intake of 4.4 mg/kg bw per day in male rats and 6.2 mg/kg bw per day in females) was identified.
在研究的前 9 个月的一部分时间里,与对照组和其他两个剂量组相比,来自最高剂量组的大鼠(雌雄)喝了更多的水。除了与对照组相比,处理动物 (所有治疗水平,但最常见于中剂量和高剂量组,并且在整个研究过程中不一致)(p < 0.05 至 p < 0.001)中排泄的细胞数量较高,在尿液分析中未观察到与治疗相关的不良反应。与对照组相比,5,000 mg/kg 组雄性大鼠(20/48 只动物,p < 0.01) 的肺炎发病率更高,500 和 5,000 mg/kg 组 (7/48 只动物,p < 0.01 和 13/48 只动物,p < 0.001)的肺气肿发生率更高。没有报告其他具有统计学意义的治疗相关效应(COT,1994b)。
在与为期 2 年的研究同时进行的一项研究中,大鼠(每组12 只动物/性别)在饮食中给予 0、50、500 或 5,000 毫克/千克亚铁氰化钠十水合物,持续 49 周(COT,1994b)。在研究结束时对血液进行采样,并分析总红细胞和白细胞计数、白细胞分类计数、血红蛋白浓度、PCV 和网织红细胞计数。在第 47-49 周从所有大鼠身上采集尿样,并分析胆红素、葡萄糖、血液、酮体和蛋白质。此外,还进行了肾功能检查。对所有大鼠进行肝、肺和脾脏检查。与对照组相比,500 和 5,000 mg/kg 组(雄性:683、533、1,517 和 4,000;雌性分别为 250、418、4,200 和 4,782 mg/kg 组,分别为 0、50、500 和 5,000 mg/kg 组)的 2 小时尿样中每小时排泄的平均细胞数有统计学意义增加,分别)。此外,与对照组相比,在 6 小时和 18 小时采集的最高治疗组中雌雄尿样浓度均具有统计学意义
总体而言,在这些研究中未观察到致癌作用,也没有观察到任何被认为具有毒理学意义的非肿瘤发现。特别是,在每天给予 0、4.5、45 或 450 毫克亚铁氰化钠/公斤体重的大鼠肾脏中,持续 49 周或 2 年,均未观察到与治疗相关的影响(COT,1994b)。然而,在为期 2 年的研究中,中剂量和高剂量动物在 2 小时尿样中的细胞排泄率经常高于对照组。这种效果在雄性和雌性中都可见,尽管它并不一致,并且参数的动物间差异通常很大。与对照组相比,低剂量组也观察到细胞排泄率增加,但仅发生 3 次。在组织病理学检查中未见肾脏不良反应,但在第一项中期研究中,雄性肾小球肾病的发生率和严重程度略有增加。然而,由于已知肾脏是亚铁氰化物中毒的靶器官,测试小组认为细胞排泄率增加表明偶尔会出现短暂的肾毒性,并发现每日不合格能值为每公斤体重50毫克(相等于雄性大鼠每天每公斤体重4.4毫克,雌性大鼠每天摄入每公斤体重6.2毫克)。
3.5.6 Reproductive and developmental toxicity
Reproductive toxicity studies
No studies available.
Developmental toxicity studies
Pregnant Crl:CD (SD) BR VAF/Plus strain rats (25 animals/group) were given sodium ferrocyanide once daily by gavage at levels of 0, 100, 500 or 1,000 mg/kg bw/day from gestational day (GD) 6 to 15 (as cited in COT, 1994b). Higher water consumption was noted for all treated groups throughout the study period (p < 0.05). There was a marginal higher number of fetuses and litters with larger dilation of the renal pelvis/ureter in the 500 (4/125 fetuses; 4/21 L) and 1,000 mg/kg (5/133 fetuses; 4/23 L) groups compared to the control group. The difference was not statistically significant and in the absence of any associated lesions and the small number of fetuses affected it was concluded by COT (1994b) that a relationship to treatment was considered not to have been proved. The Panel considered the highest dose tested 1,000 mg sodium ferrocyanide/kg bw per day as the NOAEL of this study.
Overall, there were no reproductive studies available and in one prenatal developmental toxicity study in rats (as cited in COT, 1994b) a NOAEL of 1,000 mg sodium ferrocyanide/kg bw per day (the highest dose tested) was identified.
3.5.7 Hypersensitivity, allergenicity and food intolerance
No data were available.
3.6 Discussion
Sodium, potassium and calcium ferrocyanide (E 535, 536 and 538) are anticaking agents authorised as food additives in the EU, previously evaluated by JECFA several times, the latest in 1974 (JECFA, 1975) and the SCF in 1990 (SCF, 1991). The SCF and JECFA established a group ADI of 0–0.025 mg/kg bw per day (calculated as sodium ferrocyanide) for sodium and potassium ferrocyanide, and sodium, potassium and calcium ferrocyanide, respectively.
Specifications for sodium, potassium and calcium ferrocyanide have been defined in the EU in Commission Regulation (EU) No 231/2012 and also by JECFA (2006). The purity is specified to be not less than 99% for sodium, potassium and calcium ferrocyanide.
Potassium ferrocyanide was absorbed to a limited extent from the gastrointestinal tract following oral administration to rats (Gage (1950), cited in JECFA). The majority was excreted unchanged in the faeces (approximately 95% of the dose). Ferrocyanide was detected in liver, spleen, kidney and heart/lungs. Free iron was detected in erythrocytes and free cyanide was detected in urine and expired air. The exposure to free cyanide was estimated to be less than 0.06 mg/kg bw after oral administration to rats of 10 mg 59Fe- and 14C-labelled potassium ferrocyanide per animal (Nielsen et al., 1990a). In humans, absorption was 0.25–0.42% (Nielsen et al., 1990b).
3.5.6 生殖和发育毒性
生殖毒性研究
没有研究结果。
发育毒性研究
妊娠 Crl:CD (SD) BR VAF/Plus 品系大鼠 (25 只动物/组) 从妊娠第 6 天到第 15 天以 0、100、500 或 1,000 mg/kg 体重/天的水平灌胃每天一次给予亚铁氰化钠(引自 COT,1994b)。在整个研究期间,所有治疗组的耗水量都较高 (p < 0.05)。与对照组相比,500 (4/125 个胎儿;4/21 L) 和 1,000 mg/kg (5/133 个胎儿;4/23 L) 组的胎儿和窝产仔数略高。差异没有统计学意义,在没有任何相关病变且受影响的胎儿数量少的情况下,COT (1994b) 得出结论,认为与治疗的关系尚未得到证实。小组认为,每天每公斤体重中测试的最高剂量为 1,000 毫克亚铁氰化钠,是本研究的 NOAEL。
总体而言,没有可用的生殖研究,在一项大鼠的产前发育毒性研究中(如 COT,1994b),确定了每天 1,000 毫克亚铁氰化钠/公斤体重(测试的最高剂量)的 NOAAL。
3.5.7 超敏反应、过敏性和食物不耐受
没有可用的数据。
3.6 讨论
亚铁氰化钠、钾和亚铁氰化钙(E 535、536 和 538)是欧盟认可为食品添加剂的抗结剂,之前曾多次由 JECFA 评估,最近一次是在 1974 年(JECFA,1975 年)和 1990 年(SCF,1991 年)。SCF 和 JECFA 确定了亚铁氰化钠和钾以及亚铁氰化钠、钾和亚铁氰化钙的每日每日可摄入量分别为 0-0.025 毫克/千克体重(以亚铁氰化钠计算)。
钠、钾和亚铁氰化钙的规格已在欧盟委员会法规 (EU) No 231/2012 和 JECFA (2006) 中定义。规定钠、钾和亚铁氰化钙的纯度不低于 99%。
大鼠口服亚铁氰化钾后,在有限程度上从胃肠道吸收(Gage (1950),引自 JECFA)。大多数在粪便中排泄不变 (约占剂量的 95%)。在肝脏、脾脏、肾脏和心脏/肺中检测到亚铁氰化物。在红细胞中检测到游离铁,在尿液和呼出的空气中检测到游离氰化物。每只动物口服10毫克59铁和14C标记的亚铁氰化钾后,估计摄入游离氰化物的分量低于每公斤体重0.06毫克(Nielsen等人,1990a)。在人类中,吸收率为 0.25-0.42%(Nielsen 等人,1990b)。
Potassium ferrocyanide is of low acute oral toxicity.
Treatment-related effects were observed in kidneys (higher organ weight, tubular damage and granular and calcified deposits) in rats given 0.5% and 5% sodium ferrocyanide (450 and 4,500 mg/kg bw per day) in the diet for 13 weeks (Oser, 1959; cited in JECFA (1975)). No treatment-related effects were observed in dogs given dietary sodium ferrocyanide up to 25 mg/kg bw per day for 13 weeks (Morgaridge, 1970; cited in JECFA (1975)).
Based on the available data, the Panel considered that the use of ferrocyanides as food additives is not of genotoxic concern.
No carcinogenic effects were observed in rats given 0, 50, 500 or 5,000 mg/kg sodium ferrocyanide in the diet for either 49 weeks or 2 years (COT, 1994b). However, in the 2-year study, mid- and high dose animals frequently showed a higher cell excretion rate in 2-h urine samples than did controls. Since the kidney is known to be the target organ for ferrocyanide toxicity, the Panel considered the increased cell excretion rate indicative for occasional, transient kidney toxicity and identified a NOAEL of 4.4 mg/kg bw per day in male rats and 6.2 mg/kg bw per day in females.
There were no reproductive toxicity studies available and in one prenatal developmental toxicity study in rats (as cited in COT, 1994b) a NOAEL of 1,000 mg sodium ferrocyanide/kg bw per day (the highest dose tested) was identified.
The Panel considered the excretion of a high number of cells in the urine of mid- and high-dose rats in the 2-year study as pivotal effect. Based on the lowest NOAEL for this effect of 4.4 mg sodium ferrocyanide/kg bw per day for male rats, the Panel derived an ADI of 0.044 mg sodium ferrocyanide/kg bw per day. Assuming that the toxicity of this compound is due to the ferrocyanide ion only, the Panel established a group ADI for sodium, potassium and calcium ferrocyanide of 0.03 mg/kg bw per day expressed as ferrocyanide ion. The Panel noted that at this ADI the potential amount of free cyanide released would not be of safety concern.
To assess the dietary exposure to ferrocyanides (E 535–538) from their use as food additives, the exposure was calculated based on (1) MPL in FC 12.1.1 ‘Salt’ set out in the EU legislation (defined as the regulatory maximum level exposure assessment scenario) and (2) the mean reported use levels of salt (defined as the refined exposure assessment scenario).
The Panel decided to use salt intake data from urinary excretion studies for the assessment of exposure to ferrocyanides (E 535–538) instead of the food consumption data from the EFSA Comprehensive European Food Consumption Database as dietary surveys are commonly not considered as a good source of information in the estimation of salt intake while a more accurate way of estimation of the salt intake is a calculation from the urinary excretion of sodium.
在饮食中服用0.5%和5%亚铁氰化钠(每天450至4,500毫克/公斤体重)13周的大鼠的肾脏中观察到与治疗相关的影响(器官重量增加、肾小管损伤以及颗粒状和钙化沉积物)(Oser,1959;引自JECFA(1975))。在每天饮食中给予亚铁氰化钠高达 25 毫克/公斤体重的狗,持续 13 周,未观察到与治疗相关的影响(Morgaridge,1970 年;引自 JECFA (1975))。
根据现有数据,评估小组认为使用亚铁氰化物作为食物添加剂不构成基因毒性问题。
在饮食中给予 0、50、500 或 5,000 mg/kg 亚铁氰化钠 49 周或 2 年的大鼠中未观察到致癌作用(COT,1994b)。然而,在为期 2 年的研究中,中剂量和高剂量动物在 2 小时尿样中的细胞排泄率经常高于对照组。由于已知肾脏是亚铁氰化物中毒的靶器官,因此测试小组认为细胞排泄率增加表明偶尔会出现短暂的肾毒性,结果发现雄性大鼠的每日总毒性作用水平为每公斤体重4.4毫克,雌性大鼠每日的净空毒性水平为每公斤体重6.2毫克。
没有可用的生殖毒性研究,在一项大鼠的产前发育毒性研究中(引自 COT,1994b),确定了每天 1,000 毫克亚铁氰化钠/公斤体重的 NOAEL(测试的最高剂量)。
评估小组认为,在为期 2 年的研究中,中剂量和高剂量大鼠尿液中大量细胞的排泄是关键效应。根据雄性大鼠每天每公斤体重摄入4.4毫克亚铁氰化钠的最低每日可降低预期摄入量,该小组得出每日每公斤体重摄入0.044毫克亚铁氰化钠的每日可接受摄入量。假设该化合物的毒性仅由亚铁氰化物离子引起,评估小组确定了亚铁氰化物钠、钾和钙的每日0.03毫克每日每日每日可摄入量(以亚铁氰化物离子表示)。小组指出,在此每日可接受摄入量下,释放的游离氰化物的潜在量不会构成安全问题。
为了评估亚铁氰化物 (E 535-538) 用作食品添加剂对亚铁氰化物 (E 535-538) 的膳食摄入量,根据 (1) 欧盟立法中规定的 FC 12.1.1“盐”中的 MPL(定义为监管最高水平暴露评估情景)和 (2) 盐的平均报告使用量(定义为精炼接触评估情景)计算。
评估小组决定使用尿液排泄研究中的盐摄入量数据来评估亚铁氰化物的暴露量 (E 535-538),而不是 EFSA 欧洲综合食品消费数据库中的食物消费数据,因为膳食调查通常不被认为是估计盐摄入量的良好信息来源,而估计盐摄入量的更准确方法是根据尿钠排泄量计算。
Dietary exposure to ferrocyanides was calculated based on mean and high levels consumption of salts in both the regulatory maximum level and the refined scenario.
In the MPL scenario, the exposure to ferrocyanides (E 535–538) from their use as a food additive was up to 0.009 mg/kg bw per day in children and adolescents. In the refined estimated exposure scenario, the exposure was up to 0.004 mg/kg bw per day in children and adolescents. Considering that the majority of the use levels in salt reported by Industry were for sodium ferrocyanide (E 535), these exposures would correspond approximately to 0.003 mg ferrocyanide ion/kg bw per day in children and adolescents in the refined exposure scenario.
Information from the Mintel's GNPD showed that from the salt products of subcategory ‘Seasonings’ only 13% was labelled with ferrocyanides (E 535–538) while in the exposure assessment it was assumed that 100% of the salt consumed contains the additive.
Overall, the Panel considered that the uncertainties identified indicate an overestimation of the exposure to ferrocyanides (E 535–538) as food additives in European countries for both the regulatory maximum level and the refined exposure scenario.
The Panel also noted that the refined exposure estimates are based on information provided on the reported level of use of ferrocyanides (E 535–538). If actual practice changes this refined estimates may no longer be representative and should be updated.
4 Conclusions
Considering that:
· in the refined exposure scenario estimated exposure to ferrocyanides (E 535–538) would correspond approximately to 0.003 mg ferrocyanide ion/kg bw per day in children and adolescents;
· absorption of ferrocyanides from the gastrointestinal tract was low, and there is no accumulation in human;
· ferrocyanides are of low acute toxicity and not mutagenic or carcinogenic;
· reproductive studies were not available, but a NOAEL of 1,000 mg sodium ferrocyanide/kg bw per day (highest dose tested) was identified from a prenatal developmental toxicity study;
· the kidney is the target organ for ferrocyanides toxicity as characterised by the high number of cells excreted in the urine in rats;
· 4.4 mg sodium ferrocyanide/kg bw per day was identified as the NOAEL for this effect in a chronic (2-year) study in rats;
· assuming that the toxicity of this compound is due to the ferrocyanide ion only, the Panel established a ADI for ferrocyanide ion of 0.03 mg/kg bw per day;
膳食摄入亚铁氰化物是根据监管最高水平和精制盐情景中盐的平均和高水平摄入量计算的。
在 MPL 情景中,儿童和青少年因用作食品添加剂而暴露于亚铁氰化物 (E 535-538) 的摄入量高达每天 0.009 毫克/千克体重。在精炼后的估计暴露量情景中,儿童和青少年的暴露量高达每天每公斤体重 0.004 毫克。考虑到工业界报告的盐中的大部分使用水平是亚铁氰化钠(E 535),在精制盐暴露情景下,这些暴露大约相当于儿童和青少年每天 0.003 毫克亚铁氰化物离子/千克体重。
来自英敏特 GNPD 的信息显示,在“调味品”子类别的盐产品中,只有 13% 标有亚铁氰化物(E 535-538),而在暴露评估中,假设 100% 的食用盐含有添加剂。
总体而言,评估小组认为,所确定的不确定性表明,在监管最高水平和精制盐接触情景中,欧洲国家高估了作为食品添加剂的亚铁氰化物(E 535-538)的暴露量。
评估小组还指出,精制盐的接触估计数是基于所提供的关于亚铁氰化物使用报告水平的信息(E 535-538)。如果实际做法发生变化,则此改进的估计可能不再具有代表性,应进行更新。
4 结论
考虑到:
·在精制盐暴露情景中,估计的亚铁氰化物暴露量(E 535-538)大约相当于儿童和青少年每天 0.003 毫克亚铁氰化物离子/千克体重;
·亚铁氰化物从胃肠道吸收率低,在人体内无蓄积;
·亚铁氰化物的急性毒性低,不致突变或致癌;
·没有生殖研究,但从产前发育毒性研究中确定了 1,000 毫克亚铁氰化钠/公斤体重/天(测试的最高剂量)的 NOAAL;
·肾脏是亚铁氰化物中毒的目标器官,其特征是大鼠尿液中排泄的细胞数量多;
·在一项对大鼠进行的慢性(2 年)研究中,每天 4.4 毫克亚铁氰化钠/千克体重被确定为这种效果的 NOAAL;
·假设该化合物的毒性仅由亚铁氰化物离子引起,则评估小组确定亚铁氰离子的每日可容忍摄入量为每天每公斤体重0.03毫克。
· ferrocyanides (E 535–538) are only permitted as food additives in two food categories.
The Panel concluded that ferrocyanides (E 535–538) are of no safety concern in these current authorised use and use levels.
The Panel further concluded that the available data give reason to revise the ADI of 0.025 mg sodium ferrocyanide/kg bw per day (equivalent approximately to 0.02 mg ferrocyanide ion/kg bw per day) based on a subchronic study, to a group ADI for sodium, potassium and calcium ferrocyanide of 0.03 mg/kg bw per day expressed as ferrocyanide ion.
Documentation provided to EFSA
1. Pre-evaluation document on sodium, potassium and calcium ferrocyanide (E 535, 536 and 538). Technical University of Denmark (DTU). Submitted in November 2013.
2. Extensive Literature search covering from January 1990 up to May 2018. Analytical LASER submitted in May 2018.
3. FoodDrinkEurope (FDE), 2017. Data on usage levels of sodium ferrocyanide (E 535) and potassium ferrocyanide (E 536) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6), Published 23 February 2017. Submitted to EFSA on 29 November 2017.
4. European Potato Processors’ Association (EUPPA), 2017. Data on usage levels of sodium ferrocyanide (E 535) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6), Published 23 February 2017. Submitted to EFSA on 30 November 2017.
5. European Salt Producers’ Association (EU_SALT), 2017. Data on usage levels of sodium ferrocyanide (E 535) and potassium ferrocyanide (E 536) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6), Published 23 February 2017. Submitted to EFSA on 25 November 2017.
6. Ornua, 2017. Data on usage levels of Data on usage levels of sodium ferrocyanide (E 535) and potassium ferrocyanide (E 536) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6), Published 23 February 2017. Submitted to EFSA on 29 November 2017.
7. Intersnack, 2017. Data on usage levels of potassium ferrocyanide (E 536) and calcium ferrocyanide (E 538) in foods in response to the EFSA call for food additives usage level and/or concentration data in food and beverages intended for human consumption (Batch 6), Published 23 February 2017. Submitted to EFSA on 29 November 2017.
亚铁氰化物(E 535–538)仅允许作为两类食品的食品添加剂。
评估小组得出结论,亚铁氰化物(E 535-538) 在目前的授权使用和使用水平下不存在安全问题。
评估小组进一步得出结论,现有数据有理由根据一项亚慢性研究,将每天每公斤体重0.025毫克亚铁氰化钠的每日可摄入量(相当于每天每公斤体重约0.02毫克)修订为每天每公斤亚铁氰化物钠、钾和钙的每日可摄入量为0.03毫克/公斤体重,以亚铁氰化物离子表示。
提供给欧洲食品安全局 EFSA的文件
1.关于亚铁氰化钠、钾和钙的预评价文件(E 535、536 和 538)。丹麦技术大学(DTU)。提交于2013年 11 月。
2.广泛的文献检索,涵盖从1990年 1 月到2018年5月。2018年5月提交的 Analytical LASER。
3.FoodDrinkEurope(FDE),2017年。2017年2月23日发布的响应欧洲食品安全局关于供人类消费食品和饮料中食品添加剂使用水平和/或浓度数据(第 6 批)的食品中亚铁氰化钠(E 535)和亚铁氰化钾(E 536)使用量的数据。于2017年11月29 日提交给 EFSA。
4.欧洲马铃薯加工商协会(EUPPA),2017 年。响应 EFSA 关于供人类食用的食品和饮料中食品添加剂使用水平和/或浓度数据的呼吁(第 6 批),食品中亚铁氰化钠(E 535)使用水平的数据,2017 年 2 月 23 日发布。于2017 年11 月30 日提交给EFSA。
5.欧洲盐生产商协会(EU_SALT),2017 年。2017 年 2 月 23 日发布的响应欧洲食品安全局关于供人类消费食品和饮料中食品添加剂使用水平和/或浓度数据(第 6 批)的食品中亚铁氰化钠 (E 535) 和亚铁氰化钾(E 536)使用量的数据。于2017 年 11 月 25 日提交给 EFSA。
6.Ornua,2017 年。使用水平数据 响应欧洲食品安全局关于供人类食用食品和饮料中食品添加剂使用水平和/或浓度数据(第 6 批)的呼吁,食品中亚铁氰化钠(E 535)和亚铁氰化钾(E 536)的使用水平数据,2017年2 月23 日发布。于2017年11 月29 日提交给 EFSA。
7.Intersnack,2017 年。2017 年2 月23 日发布的响应欧洲食品安全局关于供人类食用食品和饮料中食品添加剂使用水平和/或浓度数据(第 6 批)的呼吁,食品中亚铁氰化钾 (E 536) 和亚铁氰化钙 (E 538) 的使用水平数据。于 2017 年 11 月 29 日提交给 EFSA。
Notes
· 1 Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
· 2 Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
· 3 Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re-evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. OJ L 80, 26.3.2010, p. 19–27.
· 4 COM(2001) 542 final.
· 5 Food Additives in Europe 2000, Status of safety assessments of food additives presently permitted in the EU, Nordic Council of Ministers, TemaNord 2002, 560.
· 6 Call for Call for technical and toxicological data on miscellaneous food additives to be re-evaluated under the Regulation (EU) No 257/2010. Published: 11 August 2017. Available from: https://www.efsa.europa.eu/en/consultations/call/170811 Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
· 7 11–12 May 2018.
· 8 Available online: http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
· 9 Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
· 10 Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p 1.
· 11 Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16.
· 12 https://www.efsa.europa.eu/en/data/call/170223
· 13 https://www.efsa.europa.eu/en/consultations/call/170929
注释
1 2003 年 9 月 22 日欧洲议会和理事会关于动物营养添加剂的第 1831/2003 号法规 (EC)。OJ L 268,2003 年 10 月 18 日,第 29 页。
2 关于食品添加剂。OJ L 354,2008年12月31日,第16-33 页。
3 2010年3月25日欧盟委员会第257/2010号法规,根据欧洲议会和理事会关于食品添加剂的第1333/2008号法规(EC),设立了对批准的食品添加剂进行重新评估的计划。OJ L 80,2010年3月26日,第19-27页。
4 COM(2001)542最终版。
5 2000年欧洲食品添加剂,欧盟目前允许的食品添加剂安全评估状况,北欧部长理事会,TemaNord 2002,560
6 征集根据法规 (EU) No 257/2010 重新评估的其他食品添加剂的技术和毒理学数据。出版日期:2017 年 8 月 11 日。在线得到:
https://www.efsa.europa.eu/en/consultations/call/170811
http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
7 2018年5月11日至12日。
8 在线得到:
http://www.efsa.europa.eu/en/food-consumption/comprehensive-database
9 欧洲议会和理事会 2008 年 12 月 16 日关于食品添加剂的第 1333/2008 号法规 (EC)。OJ L 354,2008 年 12 月 31 日,第 16-33 页。
10 2012 年 3 月 9 日颁布的欧盟委员会第 231/2012 号法规,规定了欧洲议会和理事会第 1333/2008 号法规 (EC) 附件 II 和 III 所列食品添加剂的规格。OJ L 83,2012 年 3 月 22 日,第 1 页。
11 欧洲议会和理事会 2008 年 12 月 16 日关于食品添加剂的第 1333/2008 号法规 (EC)。OJ L 354,2008 年 12 月 31 日,第 16 页
12 https://www.efsa.europa.eu/en/data/call/170223
13 https://www.efsa.europa.eu/en/consultations/call/170929
Supporting Informati
Filename | Description |
efs25374-sup- 0001-Appendix_A- D.xlsx MS Excel, 69.7 KB | Summary of reported use levels (mg/kg or mg/L as appropriate) of E 535–538 ferrocyanides provided by industry Number and percentage of food products labelled with E 535–538 ferrocyanides out of the total number of food products present in the Mintel GNPD per food subcategory between 2013 and April 2018 Number Available data on daily sodium urinary excretion in children and adults in European countries and their estimated salt intake used for the exposure assessments Summary of total estimated exposure of ferrocyanides (E 535–538) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high exposure (mg/kg bw per day) |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
References
Basu A, Biswas D and Mukherjee A, 2013. Genotoxicity testing of two anticaking agents: sodium and potassium ferrocyanide in vitro. International Journal of Human Genetics, 13, 21–25.
Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, Elliott P; INTERSALT Co-Operative Research Group, 2013. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. American Journal of Epidemiology, 177, 1180–1192.https://doi.org/10.1093/aje/kwt066
支持信息
文件名 | 描述 |
efs25374-sup-0001-Appendix_A-D.xlsx MS Excel, 69.7 KB | 各行业提供的 E 535-538 亚铁氰化物报告使用量(mg/kg 或 mg/L 视情况而定)摘要 2013 年至 2018 年 4 月期间,标有 E 535-538 亚铁氰化物的食品数量占 Mintel GNPD 中每个食品子类别的食品总数的数量和百分比 欧洲国家儿童和成人每日尿钠排泄量及其估计盐摄入量的现有数据接触评估 在最高接触水平情景下,亚铁氰化物用作食品添加剂的总估计接触量 (E 535-538) 摘要,以及按人群和调查进行的精制盐接触评估情景:平均和高接触量(毫克/公斤体重/天) |
![]() efs25374-sup-0001-appendix_a-d.xlsx
efs25374-sup-0001-appendix_a-d.xlsx
请注意:出版商不对作者提供的任何支持信息的内容或功能负责。任何疑问(缺少内容除外)都应直接联系文章的通讯作者。
参考资料
Basu A、Biswas D 和 Mukherjee A,2013 年。两种抗结剂的遗传毒性测试:体外亚铁氰化钠和亚铁氰化钾。国际人类遗传学杂志,13, 21-25。
Brown IJ, Dyer AR, Chan Q, Cogswell ME, 上岛 H, 斯坦勒 J, 埃利奥特 P;INTERSALT 合作研究小组,2013年。从西方人群的随机尿钠浓度估计24小时尿钠排泄:INTERSALT研究。美国流行病学杂志,177,1180-1192。
https://doi.org/10.1093/aje/kwt066
COT (Committees on Toxicity Mutagenicity Carcinogenicity of Chemicals in Food, Consumer Products and the Environment), 1994a. Annual Report. Available online: http://cot.food.gov.uk/pdfs/cotcomcocannualreport1994.pdf
COT (Committee on The Toxicity of Chemicals in Food, Consumer Products and The Environment), 1994b. Potassium and Sodium Ferrocyanides. TOX 94/45.
Cotton FA, Wilkinson G, Murillo CA and Bochmann M, 1999. Advanced Inorganic Chemistry, 6th Edition. Wiley-Interscience, John Wiley & Sons, Inc, New York.
Dorazio SJ and Bruckner C, 2015. Why is there cyanide in my table salt? Structural chemistry of the anticaking effect of yellow prussiate of soda (Na4[Fe(CN)6]·10H2O). Journal of Chemical Education, 92, 150416121705004.
EFSA (European Food Safety Authority), 2007. Scientific opinion of the Scientific Committee related to uncertainties in dietary exposure assessment. EFSA Journal 2007; 5(1): 438, 54 pp. https://doi.org/10.2903/j.efsa.2007.438
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012; 10(7): 2760, 60 pp. https://doi.org/10.2903/j.efsa.2012.2760
EFSA Scientific Committee, 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009; 7(7): 1051, 22 pp. https://doi.org/10.2903/j.efsa.2009.1051
EFSA Scientific Committee, 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012; 10(3): 2579, 32 pp.
COT(食品、消费品和环境中化学品的毒性、致突变性、致癌性委员会),1994a. 年度报告。可在线获取:
http://cot.food.gov.uk/pdfs/cotcomcocannualreport1994.pdf
COT(食品、消费品和环境中化学品的毒性委员会),1994b。亚铁氰化钾和亚铁氰化钠。毒药 94/45。
Cotton FA、Wilkinson G、Murillo CA 和 Bochmann M,1999 年。高级无机化学,第 6 版。Wiley-Interscience,John Wiley & Sons, Inc,纽约。
Dorazio SJ 和 Bruckner C,2015 年。为什么我的食盐中含有氰化物?苏打黄普鲁士石 (Na4[Fe(CN)6]·10H2O) 抗结块作用的结构化学。化学教育杂志, 92, 150416121705004.
EFSA(欧洲食品安全局),2007 年。科学委员会关于膳食暴露评估不确定性的科学意见。EFSA 杂志 2007;5(1):438,54 页。
https://doi.org/10.2903/j.efsa.2007.438
EFSA ANS 小组(EFSA 食品添加剂和营养来源小组),2012 年。食品添加剂评估提交指南。EFSA 杂志 2012;10(7):2760,60 页
https://doi.org/10.2903/j.efsa.2012.2760
EFSA 科学委员会,2009 年。EFSA 开展的风险评估科学方面透明度科学委员会的指南。第 2 部分:一般原则。EFSA 杂志 2009;7(7):1051,22 页。
https://doi.org/10.2903/j.efsa.2009.1051
EFSA 科学委员会,2012a。在没有实际测量数据的情况下,EFSA 科学委员会、科学小组和单位将使用的选定默认值的指南。EFSA 杂志 2012;10(3):2579,32 页。
https://doi.org/10.2903/j.efsa.2012.2579
EFSA Scientific Committee, 2012b. Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012; 10(7): 2750, 103 pp. https://doi.org/10.2903/j.efsa.2012.2750
EFSA Scientific Committee, 2012c. Statement on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA Journal 2012; 10(3): 2578, 5 pp. https://doi.org/10.2903/j.efsa.2012.2578
European Commission (Health & Consumer Protection Directorate-General), 2001. Opinion of the Scientific Committee for Animal Nutrition on the safety of potassium and sodium ferrocyanide used as anticaking agents.
Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML, Moshfegh AJ, Kipnis V, Arab L and Prentice RL, 2015. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. American Journal of Epidemiology, 181, 473–487. https://doi.org/10.1093/aje/kwu325
Gibson RS, 2005. Principles of Nutritional Assessment. Oxford University Press, New York, US. 907 pp.
Hansen TB, Skibsted LH and Andersen HJ, 1996. The influence of anticaking agent potassium ferrocyanide and salt on the oxidative stability of frozen minced pork meat. Meat Science, 43, 135–144.
JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1970a. Toxicological evaluation of some food colours, emulsifier, stabilizers, anti-caking agents and certain other substances. FAO Nutrition Meetings Report Series No. 46A. WHO Food Additives Series, 70.36.
https://pubmed.ncbi.nlm.nih.gov/22060568/
JECFA (Thirteenth Report of the Joint FAO/WHO Expert Committee on Food Additives), 1970b. Specifications for the identity and purity of food additives and their toxicological evaluation. WHO Technical report series No. 445, FAO nutrition meeting report series No.46.
EFSA 科学委员会,2012b. 关于探索基于毒理学关注阈值 (TTC) 概念的有关可能的人类健康风险提供建议的选择的科学意见。EFSA 杂志 2012;10(7):2750,103 页。
https://doi.org/10.2903/j.efsa.2012.2750
EFSA 科学委员会,2012c。关于暴露限度方法适用于食品/饲料中添加物质中具有遗传毒性和致癌性的杂质安全性评估的声明。EFSA 杂志 2012;10(3):2578,5 页
https://doi.org/10.2903/j.efsa.2012.2578
欧洲委员会(健康与消费者保护总局),2001年。动物营养科学委员会关于用作抗结剂的亚铁氰化钾和亚铁氰化钠安全性的意见。
欧洲委员会(健康与消费者保护总局),2001年。动物营养科学委员会关于用作抗结剂的亚铁氰化钾和亚铁氰化钠安全性的意见。
https://doi.org/10.1093/aje/kwu325
吉布森 RS,2005 年。营养评估原则。牛津大学出版社,美国纽约。907 页
Hansen TB、Skibsted LH 和 Andersen HJ,1996年。抗结剂亚铁氰化钾和盐对冷冻猪肉末氧化稳定性的影响。肉类科学,43,135-144。
JECFA(粮农组织/世卫组织食品添加剂联合专家委员会),1970a。对某些食用色素、乳化剂、稳定剂、抗结块剂和某些其他物质进行毒理学评价。粮农组织营养会议报告系列第 46A 号。世卫组织食品添加剂系列,70.36。
https://pubmed.ncbi.nlm.nih.gov/22060568/
JECFA(粮农组织/世卫组织食品添加剂联合专家委员会第十三份报告),1970b。食品添加剂的特性和纯度及其毒理学评价的规范。世卫组织技术报告系列第 445号,粮农组织营养会议报告系列第 46 号。
JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1974a. Toxicological evaluation of some food additives includind anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. WHO Food Additives Series NO.5.
JECFA (Seventeenth report of the Joint FAO/WHO Expert Committee on Food Additives), 1974b. Toxicological evaluation of certain food additives with a review of general principles and of specifications. WHO Technical report series No. 539, FAO nutrition meeting report series No.53.
JECFA (Eighteenth report of the Joint FAO/WHO Expert Committee on Food Additives), 1974c. Evaluation of certain food additives. WHO Technical report series No. 557, FAO nutrition meeting report series No.54.
JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1975. Toxicological evaluation of some food colours, enzymes, flavour enhancers, thickening agents, and certain food additives. WHO Food Additives Series NO.6.
JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2000. Guidelines for the preparation of toxicological working papers for the Joint FAO/WHO Expert Committee on Food Additives. Geneva, Switzerland.
JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Monograph 1. Combined compendium of food additive specifications. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/
Ji C, Miller MA, Venezia A, Strazzullo P and Cappuccio FP, 2014. Comparisons of spot vs 24-h urine samples for estimating population salt intake: validation study in two independent samples of adults in Britain and Italy. Nutrition, Metabolism and Cardiovascular Diseases, 24, 140–147. https://doi.org/10.1016/j.numecd.2013.06.011
Kanematsu N, Hara M and Kada T, 1980. Rec assay and mutagenicity studies on metal compounds. Mutation Reserach, 77, 109–116.
https://pubmed.ncbi.nlm.nih.gov/8432042/
JECFA(粮农组织/世卫组织食品添加剂联合专家委员会),1974年a。一些食品添加剂的毒理学评价,包括抗结剂、抗菌剂、抗氧化剂、乳化剂和增稠剂。WHO 食品添加剂系列 NO.5。
JECFA (粮农组织/世界卫生组织食品添加剂联合专家委员会第十七次报告),1974b。对某些食品添加剂进行毒理学评价,并回顾一般原则和规格。世卫组织技术报告系列第 539 号,粮农组织营养会议报告系列第 53 号。
JECFA(粮农组织/世界卫生组织食品添加剂联合专家委员会第十八次报告),1974c。某些食品添加剂的评价。世卫组织技术报告系列第 557 号,粮农组织营养会议报告系列第 54 号。
JECFA(粮农组织/世卫组织食品添加剂联合专家委员会),1975 年。对某些食用色素、酶、增味剂、增稠剂和某些食品添加剂进行毒理学评价。WHO 食品添加剂系列 NO.6。
JECFA(粮农组织/世卫组织食品添加剂联合专家委员会),2000 年。为粮农组织/世卫组织食品添加剂联合专家委员会编写毒理学工作文件的指南。瑞士日内瓦。
JECFA(粮农组织/世卫组织食品添加剂联合专家委员会),2006 年。专著 1.食品添加剂规格综合纲要。可在线获取:
http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/
Ji C、Miller MA、Venezia A、Strazzullo P 和 Cappuccio FP,2014 年。用于估计人群盐摄入量的现场样本与 24 小时尿液样本的比较:英国和意大利两个独立成人样本的验证研究。营养、代谢和心血管疾病,24,140-147。
https://doi.org/10.1016/j.numecd.2013.06.011
Kanematsu N、Hara M 和 Kada T,1980 年。金属化合物的 Rec 测定和致突变性研究。突变研究,77,109-116。
https://pubmed.ncbi.nlm.nih.gov/8432042/
Kawasaki T, Itoh K, Uezono K and Sasaki H, 1993. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clinical and Experimental Pharmacology and Physiology, 20, 7–14.
Kruse JM and Thibault LE, 1973. Determination of Free Cyanide in Ferrocyanides and Ferricyanides. Analytical Chemistry, 45, 2260–2261. Available online: Go to ISI://A1973R098900025
Li YL, Liu ZF, Xie LS, Kong L and Liu SP, 2006. Determination of potassium ferrocyanide in foods by resonance Rayleigh scattering method with basic triaminotriphenylmethane dyes. Chinese Chemical Letters, 17, 1595–1598. Available online: Go to ISI://000243628000020
Li YL, Liu ZF, Xie LS, Kong L and Liu SP, 2007. Determination of potassium ferrocyanide in foods by resonance Rayleigh scattering method with double-charged triaminotriphenylmethane dyes. Chinese Journal of Chemistry, 25, 947–952. Available online: Go to ISI://000248026100014
McLean RM, Farmer VL, Nettleton A, Cameron CM, Cook NR and Campbell NRC, TRUE Consortium (International Consortium for Quality Research on Dietary Sodium/Salt), 2017. Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: a systematic literature review. The Journal of Clinical Hypertension (Greenwich), 19, 1214–1230. https://doi.org/10.1111/jch.13148
Nguyen MV, Thorarinsdottir KA, Thorkelsson G, Gudmundsdottir A and Arason S, 2012. Influences of potassium ferrocyanide on lipid oxidation of salted cod (Gadus morhua) during processing, storage and rehydration. Food Chemistry, 131, 1322–1331.
Nielsen P, Dresow B, Fischer R and Heinrich HC, 1990a. Bioavailability of iron and cyanide from 59Fe- and 14C-labelled hexacyanoferrates(II) in rats. Z Naturforsch C, 45, 681–690. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2400471
Nielsen P, Dresow B, Fischer R and Heinrich HC, 1990b. Bioavailability of iron and cyanide from oral potassium ferric hexacyanoferrate(II) in humans. Arch Toxicology, 64, 420–422.
Kawasaki T、Itoh K、Uezono K 和 Sasaki H,1993 年。一种估计成人第 2 晨排尿尿标本中 24 小时尿钠和尿钾排泄的简单方法。临床和实验药理学与生理学,20,7-14。
Kruse JM 和 Thibault LE,1973 年。亚铁氰化物和铁氰化物中游离氰化物的测定。分析化学, 45, 2260–2261.在线提供: 前往 ISI://A1973R098900025
Li YL, Liu ZF, Xie LS, Kong L 和 Liu SP, 2006.用碱性三氨基三苯基甲烷染料的共振瑞利散射法测定食品中的亚铁氰化钾。中国化学快报, 17, 1595–1598.在线提供: 前往 ISI://000243628000020
Li YL、Liu ZF、Xie LS、Kong L 和 Liu SP,2007 年。用双电荷三氨基三苯基甲烷染料的共振瑞利散射法测定食品中的亚铁氰化钾。中国化学, 25, 947–952.在线提供: 前往 ISI://000248026100014
McLean RM、Farmer VL、Nettleton A、Cameron CM、Cook NR 和 Campbell NRC,TRUE Consortium(膳食钠/盐质量研究国际联盟),2017 年。使用食物频率问卷和 24 小时尿钠排泄评估膳食钠摄入量:系统文献综述。临床高血压杂志(格林威治),19,1214-1230。
https://doi.org/10.1111/jch.13148
Nguyen MV、Thorarinsdottir KA、Thorkelsson G、Gudmundsdottir A 和 Arason S,2012 年。亚铁氰化钾对盐渍鳕鱼 (Gadus morhua) 加工、储存和再水化过程中脂质氧化的影响。食品化学, 131, 1322–1331.
尼尔森 P、Dresow B、Fischer R 和 Heinrich HC,1990a。大鼠 59Fe 和 14C 标记的六氰基铁酸盐 (II) 中铁和氰化物的生物利用度。Z Naturforsch C,45,681-690。在线提供
http://www.ncbi.nlm.nih.gov/pubmed/2400471
Nielsen P、Dresow B、Fischer R 和 Heinrich HC,1990 年b。人类口服六氰基铁酸铁钾 (II) 中铁和氰化物的生物利用度。拱门毒理学,64,420-422。
Nishioka H, 1975. Mutagenic activities of metal compounds in bacteria. Mutation Research, 31, 185–189. Available online: http://www.ncbi.nlm.nih.gov/pubmed/805366
Olivier P and Marzin D, 1987. Study of the genotoxic potential of 48 inorganic derivatives with the SOS chromotest. Mutation Research, 189, 263–269. Available online: http://www.ncbi.nlm.nih.gov/pubmed/3118206
Perrin DD, 1969. Dissociation constants of inorganic acids and bases in aqueous solutions. Pure and Applied Chemistry, 20, 133–236.
Roberts RF and Wilson RH, 1968. The determination of ferrocyanide and related compounds in commercial sodium chloride. Analyst, 63, 237–243.
SCF (Scientific Committee for Food), 1991. Reports from the Scientific Committee for Food (25th series). Opinion expressed 1990. Food - science and techniques.
SCF (Scientific Committee for Food), 2001. Guidance on submissions for food additive evaluations by the Scientific Committee on Food. SCF/CS/ADD/GEN/26 Final. 12 July 2001.
Soo Lim H, Young Hwang J, Choi E, Lee G, Sun Yoon S and Kim M, 2018. Development and validation of HPLC method for the determination of ferrocyanide ion in food grade salts. Food Chemistry 239, 1167–1174. https://doi.org/10.1016/j.foodchem.2017.07.070
Stolzenberg AM, 2005. Iron compounds. In: Kirk-Othmer Encyclopedia of Chemical Technology, 5th edition. Wiley-Interscience, John Wiley & Sons, Inc., Hoboken. pp. 530–561.
Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC and Willett WC, 2017. Reproducibility of urinary biomarkers in multiple 24-h urine samples. The American Journal of Clinical Nutrition, 105, 159–168. https://doi.org/10.3945/ajcn.116.139758. Epub 2016 Nov 9.
西冈 H,1975 年。细菌中金属化合物的诱变活性。突变研究,31,185-189。可在线获取:
http://www.ncbi.nlm.nih.gov/pubmed/805366
Olivier P 和 Marzin D,1987 年。使用 SOS chromotest 研究 48 种无机衍生物的遗传毒性潜力。突变研究,189,263-269。可在线获取:
http://www.ncbi.nlm.nih.gov/pubmed/3118206
Perrin DD,1969 年。无机酸和碱在水溶液中的解离常数。纯粹与应用化学, 20, 133–236。
Roberts RF 和 Wilson RH,1968 年。测定商品氯化钠中的亚铁氰化物和相关化合物。分析师,63,237-243。
SCF(食品科学委员会),1991 年。食品科学委员会的报告(第25 辑)。1990 、年表达的意见。食科学和技术。
SCF(食品科学委员会),2001 年。食品科学委员会提交的食品添加剂评估指南。SCF/CS/ADD/GEN/26 最终版。2001 年 7 月 12 日。
Soo Lim H、Young Hwang J、Choi E、Lee G、Sun Yoon S 和 Kim M,2018 年。用于测定食品级盐中亚铁氰化物离子的 HPLC 方法的开发和验证。食品化学 239, 1167 - 1174。
https://doi.org/10.1016/j.foodchem.2017.07.070
Stolzenberg AM,2005 年。铁化合物。收录于:Kirk-Othmer 化学技术百科全书,第 5 版。Wiley-Interscience, John Wiley & Sons, Inc., 霍博肯。第 530-561 页。
Sun Q、Bertrand KA、Franke AA、Rosner B、Curhan GC 和 Willett WC,2017 年。多个 24 小时尿液样本中尿液生物标志物的可重复性。美国临床营养学杂志, 105, 159–168。
https://doi.org/10.3945/ajcn.116.139758. Epub 2016 Nov 9.
Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H and Hashimoto T, 2002. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. Journal of Human Hypertension, 16, 97–103.
TemaNord, 2002. Food Additives in Europe 2000 - Status of safety assessments of food additives presently permitted in the EU.
Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, Sempos CT, Gillespie CD and Burt VL, 2013. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. Journal of Nutrition, 143, 1276–1282. https://doi.org/10.3945/jn.113.175927
WHO (World Health Organization), 2011. Strategies to monitor and evaluate population sodium consumption and sources of sodium in the diet. Report of joint technical meeting convened by WHO and the Government of Canada. Canada, October 2010. 40 pp.
Wong-Chong GM, Nakles DV and Luthy RG, 2006. Manufacture and the Use of Cyanide. Cyanide in water and soil. Chemistry, Risk and Management. Taylor & Francis Group. pp. 41–55.
Yamane T, Isawa M and Osada S, 2006. Simple, rapid and sensitive determination of hexacyanoferrate(II) (ferrocyanide) in salts by flow injection system utilizing anion exchange column as an integrated field for sensitive detection reaction and sepration/preconcentration. Nippon Kaisui Gakkaishi, 60, 352–357.
Tanaka T、Okamura T、Miura K、Kadowaki T、Ueshima H、Nakagawa H 和 Hashimoto T,2002 年。一种使用随意尿液标本估计人群 24 小时尿钠和尿钾排泄的简单方法。人类高血压杂志,16,97-103。
TemaNord,2002 年。2000 年欧洲食品添加剂 - 欧盟目前允许的食品添加剂安全评估状况。
Wang CY、Cogswell ME、Loria CM、Chen TC、Pfeiffer CM、Swanson CA、Caldwell KL、Perrine CG、Carriquiry AL、Liu K、Sempos CT、Gillespie CD 和 Burt VL,2013 年。在 24 小时校准研究中,钠、钾和氯的尿液排泄量(但不包括碘)的排泄量因收集时间而异。营养学杂志, 143, 1276–1282。
https://doi.org/10.3945/jn.113.175927
WHO(世界卫生组织),2011 年。监测和评估人群钠摄入量和饮食中钠来源的策略。世卫组织和加拿大政府召开的联合技术会议报告。加拿大,2010 年 10 月。40 页。
Wong-Chong GM、Nakles DV 和 Luthy RG,2006 年。氰化物的制造和使用。水和土壤中的氰化物。化学、风险和管理。泰勒和弗朗西斯集团。第 41-55 页。
Yamane T、Isawa M 和 Osada S,2006 年。利用阴离子交换柱作为灵敏检测反应和分离/预浓缩的集成区域,通过流动进样系统简单、快速、灵敏地测定盐中的六氰基铁酸盐 (II)(亚铁氰化物)。日本海水学会,60,352-357。
Abbreviations缩写
ADI
acceptable daily intake 每日容许摄入量
ANS
EFSA Scientific Panel on Food Additives and Nutrient Sources added to Food EFSA 食品添加剂和食品中添加营养来源科学小组
BIBRA
British Industrial Biological Research Association 英国工业生物学研究协会
bw
body weight 体重
CAS
Chemical Abstracts Service化学摘要服务编号
CONTAM
EFSA Panel on Contaminants in Food Chain 欧洲食品安全局食物链污染物小组
COT
UK Committees on the Toxicity of Chemicals in Food, Consumer Products and the Environment 英国食品、消费品和环境中化学品毒性委员会
CV
crystal violet 结晶紫
EINECS
European Inventory of Existing Chemical Substances 欧洲现有化学物质清单
EUPPA
European Potato Processors’ Association 欧洲马铃薯加工商协会
EU_SALT
European Salt Producers’ Association 欧洲盐生产商协会
EV
ethyl violet 乙基紫
FAO
Food and Agriculture Organization of the United Nations 联合国粮食及农业组织
FCs
food categories 食物类别
CS
food categorisation system 食物分类系统
FDE
Food Drink Europe 欧洲餐饮组织
FFA
free fatty acids 游离脂肪酸
FI
flow injection 流动注射
GD
gestational day 妊娠期 天
GNPD
Global New Products Database 全球新产品数据库
HPLC
high-performance liquid chromatography 高效液相色谱
IG
iodine green 碘绿
JECFA
Joint FAO/WHO Expert Committee on Food Additives 世界粮农组织/卫生组织食品添加剂联合专家委员会
LOD
limit of detection 检测极限
LOQ
limit of quantification 定量限制
MeG
methyl green 甲基绿
MPL
maximum permitted level 最大使用量
MS
mass spectrometry 质谱分析
MV
methyl violet 甲基紫
NDA
EFSA Panel on Dietetic Products, Nutrition and Allergies 欧洲食品安全局饮食产品、营养和过敏组
NOAEL
no observed adverse effect level 未观察到不良反应水平
OECD
Organisation for Economic Co-operation and Development 经济合作与发展组织
PCV
packed cell volume 细胞压积
RBC
red blood cell 红细胞
RRS
resonance Rayleigh scattering 共振瑞利散射
RSD
relative standard deviation 相对标准偏差
SCAN
Scientific Committee for Animal Nutrition 动物营养科学委员会
SCF
Scientific Committee on Food 食物科学委员会
TBARS
thiobarbituric acid-reactive substances 硫代巴比妥酸反应物质
TemaNord
is a publishing series for results of the often research-based work that working groups or projects under Nordic Council of Ministers have put in motion 是一个出版系列,汇集了北欧部长理事会下属的工作组或项目开展的通常基于研究的工作成果
WBC
white blood cell 白血球,白细胞
WBR
whole body retention 全身潴留
WG
Working Group 工作组
WHO
World Health Organization 世界卫生组织
Appendix A – Summary of reported use levels (mg/kg or mg/L as appropriate) of E 535–538 ferrocyanides provided by industry
Appendix A can be found in the online version of this output (‘Supporting information’ section).
附录 A ——行业提供的E 535-538亚铁氰化物报告使用水平摘要(mg/kg 或mg/L视情况而定)
附录 A 可以在此输出的在线版本(“支持信息”部分)中找到。
Appendix B – Number and percentage of food products labelled with E 535–538 ferrocyanides out of the total number of food products present in the Mintel GNPD per food subcategory between 2013 and April 2018
Appendix B can be found in the online version of this output (‘Supporting information’ section).
附录 B ——2013年至2018年4月期间,在Mintel GNPD中每个食品子类别的食品总数中,标有E 535-538 亚铁氰化物的食品数量和百分比。
附录 B 可以在此输出的在线版本(“支持信息”部分)中找到。
Appendix C – Number Available data on daily sodium urinary excretion in children and adults in European countries and their estimated salt intake used for the exposure assessments
Appendix C can be found in the online version of this output (‘Supporting information’ section).
附录 C ——数量 欧洲国家儿童和成人每日尿钠排泄量及其用于暴露评估的评估盐摄入量的可用数据。
附录 C 可以在此输出的在线版本(“支持信息”部分)中找到。
Appendix D – Summary of total estimated exposure of ferrocyanides (E 535–538) from their use as food additives for the maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and high exposure (mg/kg bw per day)
Appendix D can be found in the online version of this output (‘Supporting information’ section).
附录 D ——在最高水平接触情景和精制盐接触评估情景下,亚铁氰化物(E 535-538)用作食品添加剂的总估计接触量摘要。
附录 D 可以在此输出的在线版本(“支持信息”部分)中找到。
Citing Literature
Number of times cited according to CrossRef: 4
Huan Li, Elsayed Oraby, Jacques Eksteen, Wenhao Xie, Jing Gu, Haoran Yuan, Extraction of precious metals from waste printed circuit boards using alkaline ferricyanide solutions: An exploratory study, Minerals Engineering, 10.1016/j.mineng.2024.109144, 222, (109144), (2025).
Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa, Ruud Woutersen, Gabriele Aquilina, Georges Bories, Carlo Nebbia, Jürgen Gropp, Jaume Galobart, Maria Vittoria Vettori, Montserrat Anguita, Jordi Ortuño Casanova, Angelica Amaduzzi, Matteo Lorenzo Innocenti, Safety and efficacy of feed additives consisting of sodium ferrocyanide and potassium ferrocyanide for all animal species (Eusalt a.i.s.b.l.), EFSA Journal, 10.2903/j.efsa.2023.7960, 21, 4, (2023).
Vasileios Bampidis, Giovanna Azimonti, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa, Ruud Woutersen, Gabriele Aquilina, Georges Bories, Carlo Nebbia, Jürgen Gropp, Jaume Galobart, Maria Vittoria Vettori, Montserrat Anguita, Jordi Ortuño Casanova, Angelica Amaduzzi, Matteo Lorenzo Innocenti, Safety and efficacy of a feed additive consisting of potassium ferrocyanide for all animal species (K + S KALI GmbH)* , EFSA Journal, 10.2903/j.efsa.2023.7953, 21, 6, (2023).
Telmo da Silva Lopes, Paula Dias, Ricardo Monteiro, António Vilanova, Dzmitry Ivanou, Adélio Mendes, A 25 cm2 Solar Redox Flow Cell: Facing the Engineering Challenges of Upscaling, Advanced Energy Materials, 10.1002/aenm.202102893, 12, 5, (2021).
引用文献
根据 CrossRef 的引用次数:4
环利,埃勒萨德·奥拉比,雅克·埃克斯坦,谢文浩,顾静,袁昊然,使用碱性铁氰化物溶液从废弃印刷电路板中提取贵金属:一项探索性研究,矿物工程, 10.1016/j.mineng.2024.109144, 222, (109144), (2025).
班皮迪斯、乔瓦娜·阿兹蒙蒂、玛丽亚·德·卢尔德·巴斯托斯、亨里克·克里斯滕森、比尔吉特·杜塞蒙德、莫伊卡·杜尔贾瓦、玛丽琳·库巴、玛尔塔·洛佩斯-阿隆索、塞昆迪诺·洛佩斯·普恩特、弗朗西斯卡·马尔孔、巴尔塔萨·梅奥、阿莱娜·佩乔娃、玛丽安娜·佩特科娃、费尔南多·拉莫斯、约兰达·桑斯、罗伯托·爱德华多·维拉、鲁德·沃特森、加布里埃尔·阿奎利娜、乔治·博里斯、卡洛·内比亚、尤尔根·格罗普、豪梅·加洛巴特、玛丽亚·维多利亚·维托里、蒙特塞拉特·安吉塔、乔迪·奥尔图尼奥·卡萨诺瓦、安杰丽卡·阿马杜齐、马泰奥·洛伦佐·伊诺森蒂,由亚铁氰化钠和亚铁氰化钾组成的饲料添加剂对所有动物物种的安全性和有效性(Eusalt a.i.s.b.l.),EFSA 杂志,10.2903/j.efsa.2023.7960,21,4,(2023)
瓦西里奥斯·班皮迪斯、乔瓦娜·阿兹蒙蒂、玛丽亚·德·卢尔德·巴斯托斯、亨里克·克里斯滕森、比尔吉特·杜塞蒙德、莫伊卡·杜尔贾瓦、玛丽琳·库巴、玛尔塔·洛佩斯-阿隆索、塞昆迪诺·洛佩斯·普恩特、弗朗西斯卡·马尔孔、巴尔塔萨·梅奥、阿莱娜·佩霍娃、玛丽安娜·佩特科娃、费尔南多·拉莫斯、约兰达·桑斯、罗伯托·爱德华多·维拉、鲁德·沃特森、加布里埃尔·阿奎利纳、乔治·博里斯、卡洛·内比亚、尤尔根·格罗普、豪姆·加洛巴特、玛丽亚·维多利亚·维托里、蒙特塞拉特·安吉塔、乔迪·奥尔图尼奥·卡萨诺瓦、 安杰丽卡·阿马杜齐,马泰奥·洛伦佐·因诺森蒂,由亚铁氰化钾组成的饲料添加剂对所有动物物种的安全性和有效性 (K + S KALI GmbH)*,EFSA 杂志,10.2903/j.efsa.2023.7953,21,6,(2023)。
特尔莫·达·席尔瓦·洛佩斯、保拉、里卡多、安东尼奥、德米特里·伊万诺夫、阿德利奥·门德斯,25 cm2 太阳能氧化还原流动电池:面对放大的工程挑战,先进能源材料,10.1002/aenm.202102893,12,5,(2021)。
中英对照:![]() EU重新评价食品添加剂亚铁氰化钠(E 535)、亚铁氰化钾(E 536) 和亚铁氰化钙(E 538)安全性的科学意见.pdf
EU重新评价食品添加剂亚铁氰化钠(E 535)、亚铁氰化钾(E 536) 和亚铁氰化钙(E 538)安全性的科学意见.pdf
中国评估:![]() 国家食品安全风险评估专家委员会食品添加剂亚铁氰化物(亚铁氰化钾和亚铁氰化钠)风险评估(摘要).pdf
国家食品安全风险评估专家委员会食品添加剂亚铁氰化物(亚铁氰化钾和亚铁氰化钠)风险评估(摘要).pdf